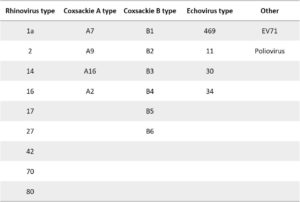
Reactivity testing was carried out by indirect fluorescent-antibody (FA) at 10 µg/ml.
Mouse Anti-Enterovirus Pan VP3 Antibody (3654)
Price range: $454.04 through $1,139.31 excl. VAT
Mouse monoclonal anti Enterovirus Pan VP3 antibody, Ideal for use in ELISA and immunofluorescence.
MOUSE ANTI-ENTEROVIRUS PAN VP3 ANTIBODY (3654)
Mouse anti Enterovirus Pan VP3 antibody is specific for Enterovirus VP3 and has been developed for use in ELISA and immunofluorescence.
PRODUCT DETAILS – MOUSE ANTI-ENTEROVIRUS PAN VP3 ANTIBODY (3654)
- Mouse anti Enterovirus Pan VP3. Reactive with Rhinoviruses, Coxsackie B viruses, ECHO viruses, Coxsackie A viruses, EV71 and Poliovirus. It is Negative for Influenza A, Influenza B, RSV, Adenovirus, Para 1, Para 2, Para 3, Chlamydia pneumoniae, Mycoplasma pneumoniae and Hepatitis A virus.
- Purified preparations consist of >90% pure mouse monoclonal antibody purified from ascites fluid or culture medium by protein A chromatography or sequential differential precipitations.
- Presented in PBS pH7.2 with 0.1% sodium azide.
- For use in ELISA and immunofluorescence.
- Can be used with Goat anti mouse IgG HRP and PanBlock ELISA Blocking Buffer.
BACKGROUND
Enteroviruses (EV) are single-stranded RNA viruses belonging to the Picornaviridae family and are the smallest, non-enveloped viruses known to infect both humans and animals. They are common seasonal viruses that are associated with a variety of diseases. They are approximately 25-30 nm in diameter, and icosahedral in shape. The viruses are non-enveloped, and the virions are relatively simple, consisting of a protein capsid surrounding a single-stranded, positive sense RNA genome. The genome has approximately 7500 nucleotides, and contains a single open reading frame that encodes a polyprotein which is then processed to yield the structural (i.e., capsid) proteins VP1, VP2, VP3, and VP4 and the non-structural proteins.
These viruses are spread primarily through the fecal–oral route, but some species can be spread through respiratory secretions (e.g., EV-D68 and rhinovirus). The average incubation period for enteroviral contagious is from 3 – 10 to 30 days. The virus, after replicating and breaking the gastrointestinal tract barrier, is transmitted via the blood stream to every organ of the body. EV shows tropism towards organs like the heart, skin, and in particular the central nervous system. It has been shown that infected people excrete large quantities of the virus in faeces for a period of even 16 weeks. Most human EV infections are either asymptomatic or result in mild disease. Periodically, EV are associated with outbreaks of more serious disease, resulting in considerable morbidity and occasionally in significant mortality.
As yet, no effective EV-specific antiviral treatments are available, and vaccines are available only against polioviruses. Ongoing experience with EV71 outbreaks in the Asia-Pacific region has demonstrated that co-infections with other EV and indeed viruses belonging to other families, is common and raises the possibility that some co-infections can increase the severity of disease and change the clinical presentation.
EV have been shown to possess major conformational neutralizing epitopes on both the VP2 and VP3 capsid proteins making them ideal targets for anti-virals and neutralising antibodies have been identified which block a receptor-binding site on EV71 VP3 (Jia et al., 2017).
REFERENCES
- Enterovirus surveillance guidelines. Guidelines for enterovirus surveillance in support of the Polio Eradication Initiative. World Health Organization 2015.
- Jia et al. (2017). Effective in vivo therapeutic IgG antibody against VP3 of enterovirus 71 with receptor-competing activity. Sci Rep. 7:46402.
- Factsheet about enteroviruses. (2010). European Centre for Disease Prevention and Control (ECDC),