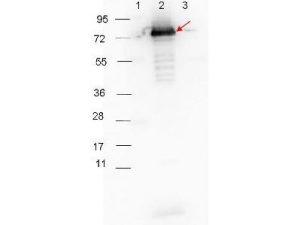
RABBIT ANTI-BORRELIA BURGDORFERI SENSU STRICTO (B31) SURFACE LIPOPROTEIN P27 ANTIBODY
This is a polyclonal antibody, prepared against Surface Lipoprotein P27 from the spirochete B. burgdorferi, for use in ELISA and western blotting applications.
PRODUCT DETAILS – RABBIT ANTI-BORRELIA BURGDORFERI SENSU STRICTO (B31) SURFACE LIPOPROTEIN P27 ANTIBODY
- Rabbit anti-B. burgdorferi sensu stricto Surface Lipoprotein P27 polyclonal IgG antibody (strain B31).
- Greater than 95% purity by SDS-PAGE and buffered in 0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2.
BACKGROUND
Strain B31 is the type strain (ATCC 35210) for this organism and was derived by limited dilutional cloning from the original Lyme-disease tick isolate obtained by A. Barbour (Johnson, et al., 1984).
The p27 gene is located on a linear plasmid of a size of approximately 55 kb and encodes a basic protein of 248 amino acids with a typical prokaryotic leader sequence of 17 amino acid residues at the N-terminus of the proposed translation product, as confirmed by Northern and Western blot analysis (Reindl, et al., 1993).
Spirochetal outer surface proteins (Osps) play a major role in both protection from and pathogenesis of Lyme disease. All Osps (OspA to OspF and P27) have been shown to be lipoproteins, containing hydrophobic N-terminal domains and putative cleavage sites (Leu-X-Y-Z-Cys) thought to be recognized by a lipoprotein signal peptidase (Brandt, et al., 1990).
Borrelia spirochetes are unique among gram-negative (diderm or double-membrane) bacteria in their abundance of surface-displayed lipoproteins, some of which play important roles in the pathogenesis of Lyme disease and relapsing fever. There is evidence that Borrelia lipoproteins are specifically targeted to the bacterial surface, but that they can be retained in the periplasm by sequence-specific signals (Schulze & Zückert, 2006).
REFERENCES
- Brandt, M. E., Riley, B. S., Radolf, J. D. & Norgard, M. V., 1990. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect. Immun., Volume 58, p. 983–991.
- Johnson, R.C., et al. 1984. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int J Syst Bacteriol, 34, pp. 496–497.
- Reindl, M., Redl, B. & Stöffler, G., 1993. Isolation and analysis of a linear plasmid-located gene of Borrelia burgdorferi B29 encoding a 27 kDa surface lipoprotein (p27) and its overexpression in Escherichia coli. Mol. Microbiol., 8(6), pp. 1115-24.
- Schulze, R. J. & Zückert, W. R., 2006. Borrelia burgdorferi lipoproteins are secreted to the outer surface by default. Mol. Microbiol., 59(5), pp. 1473 – 1484.