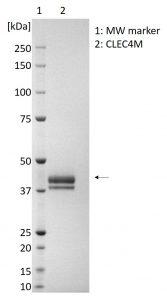
HUMAN CLEC4M (L-SIGN), HIS-TAG
Recombinant CLEC4M protein produced in HEK293 cells and purified from culture supernatant. Protein contains an N-terminal 6x His-tag.
PRODUCT DETAILS – HUMAN CLEC4M (L-SIGN), HIS-TAG
- Recombinant CLEC4M protein produced from HEK293 cells (NCBI Accession Number: XP_006722676.1).
- Includes amino acids 43-371 and an N-terminal His-tag.
- Greater then 95% purity by SDS-PAGE and buffered in DPBS, pH7.4.
BACKGROUND
C-type lectin domain family 4, member M (CLEC4M), also known as L-SIGN, is a type II integral membrane protein that has 77% amino acid identity to DC-SIGN. CLEC4M is expressed predominantly on endothelial cells of liver and lymph nodes but not on dendritic cells. The protein is organized into three distinct domains: an N-terminal transmembrane domain, a tandem-repeat neck domain and C-type lectin carbohydrate recognition domain. The extracellular region consisting of the C-type lectin and neck domains has a dual function as a pathogen recognition receptor and a cell adhesion receptor by binding carbohydrate ligands on the surface of microbes and endogenous cells. The neck region is important for homo-oligomerization which allows the receptor to bind multivalent ligands with high avidity. Variations in the number of 23 amino acid repeats in the neck domain of this protein are common and have a significant impact on ligand binding ability.
CLEC4M is believed to be involved in peripheral immune surveillance in the liver, mediating endocytosis of pathogens for degradation in lysosomal compartments. It appears to selectively recognize and bind a variety of viral surface glycoproteins containing high mannose N-linked oligosaccharides in a calcium-dependent manner, including HIV-1 gp120, HIV-2 gp120, SIV gp120, Ebola virus glycoproteins, HCV E2, and human SARS coronavirus protein S, as well as the cellular adhesion protein ICAM3.
CLEC4M likley plays an important role in establishing HIV infection by enhancing trans-infection of CD4( )T cells in regional lymph nodes. It is also a liver-specific receptor for HCV envelope glycoprotein E2, and both CLEC4M and DC-SIGN may play critical roles in viral pathogenesis and tissue tropism (Gardner, et al., 2003). Its polymorphism may also affect HCV loads and contribute to HCV replication efficacy (Lozach, et al., 2004). It is also a cofactor for cellular entry by Ebola virus (Alvarez, et al., 2002) and a SARS-CoV binding receptor; homozygosity for CLEC4M is believed to play a protective role during SARS infection (Chan, et al., 2006). CLEC4M and DC-SIGN can also function as attachment receptors for Sindbis (SB) virus, an arbovirus of the Alphavirus genus (Klimstra, et al., 2003).
References
- Alvarez, C. P. et al., 2002. C-type lectins mediate cellular entry by Ebola virus in cis and in trans. J Virol. , 76(13), pp. 6841-4.
- Chan, V. S. et al., 2006. Homozygous L-SIGN (CLEC4M) plays a protective role in SARS coronavirus infection. Nature Genetics, Volume 38, p. 38.
- Gardner, J. P. et al., 2003. L-SIGN (CD 209L) is a liver-specific capture receptor for hepatitis C virus. Proc Natl Acad Sci U S A., 100(8), p. 4498–4503.
- Klimstra, W. B. et al., 2003. DC-SIGN and L-SIGN Can Act as Attachment Receptors for Alphaviruses and Distinguish between Mosquito Cell- and Mammalian Cell-Derived Viruses. J Virol., 77(22), pp. 12022-12032.
- Lozach, P. Y. et al., 2004. C-type Lectins L-SIGN and DC-SIGN Capture and Transmit Infectious Hepatitis C Virus Pseudotype Particles. The Journal of Biological Chemistry, Volume 279, pp. 32035-32045.
- Lozach, P. Y., Burleigh, L., Staropoli, I. & Amara, A., 2007. The C type lectins DC-SIGN and L-SIGN: receptors for viral glycoproteins. Methods Mol. Biol. , Volume 379, p. 51–68.