While the 2015/16 Zika epidemic no longer makes the headlines, there remains a crucial need to accurately and reliably diagnose Zika virus (ZIKV) infection to identify and manage further outbreaks. However, high levels of cross-reactivity among cocirculating flaviviruses have plagued immunoassays with false-positive results, that make surveillance and diagnosis of the disease challenging. A recent paper by Tedder and colleagues describes the development of a double-antigen binding assay (DABA) and reverse IgG capture ELISA for the detection of ZIKV and was compared with a commercial indirect capture assay. Here we discuss the design and performance of these assays, and the effect of quenching methods to ablate cross-reacting antibodies.
Assay formats
First, lets re-cap some of the formats used to design ELISAs.
Conventional serology ELISAs use an anti-human IgG/IgM antibody conjugated to an enzyme or tag that produces a detectable and quantifiable signal upon binding to human IgG/IgM that is bound to an antigen immobilized in the well (indirect ELISA), or bound by an immobilised antibody (capture ELISA). Unlike conventional formats, double antigen binding assays (DABA) use an antigen sandwich, with the second part of the sandwich being an antigen conjugated to an enzyme to visualise specific detection.
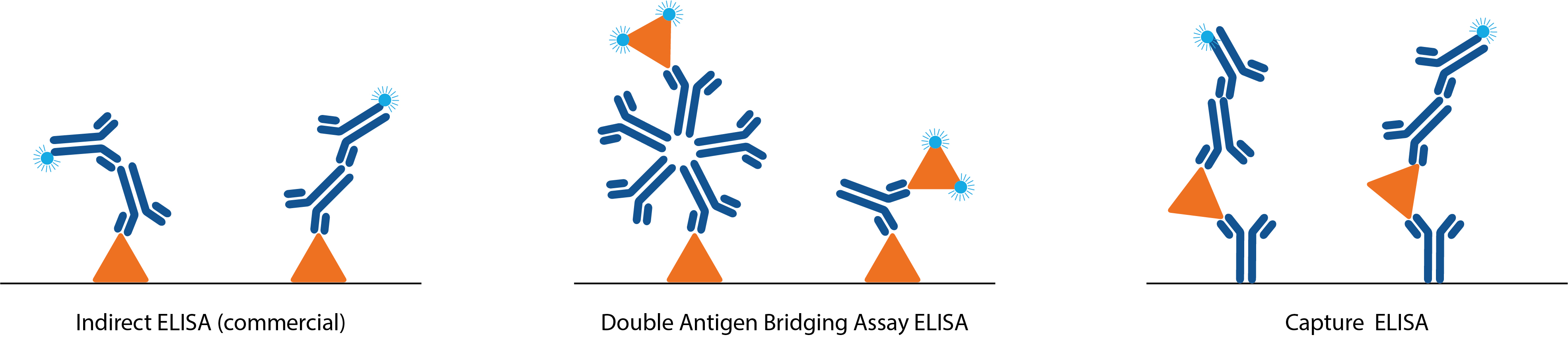
So why use the DABA format? DABAs pose several advantages to conventional ELISAs:
- They exhibit very high specificity, with minimal cross-reactivity to circulating irrelevant antibodies. DABA assays also detect higher avidity antibodies, resulting in reduced binding of lower avidity cross-reactive antibodies.
- DABAs are total antibody assays, meaning that they allow for the detection of all immunoglobulin isotypes and classes – maximising sensitivity.
- DABA formats are not species-specific, making them ideal candidates for immunological studies in animals.
The Study
To investigate the effect of using different formats, 3 different assays were tested for comparison: a commercial indirect capture ELISA was compared with a DABA assay and IgG capture ELISA developed by Tedder and colleagues. A novel method of antibody ‘quenching’ was then used to investigate changes in assay specificity.
Initial Cross-Reactivity
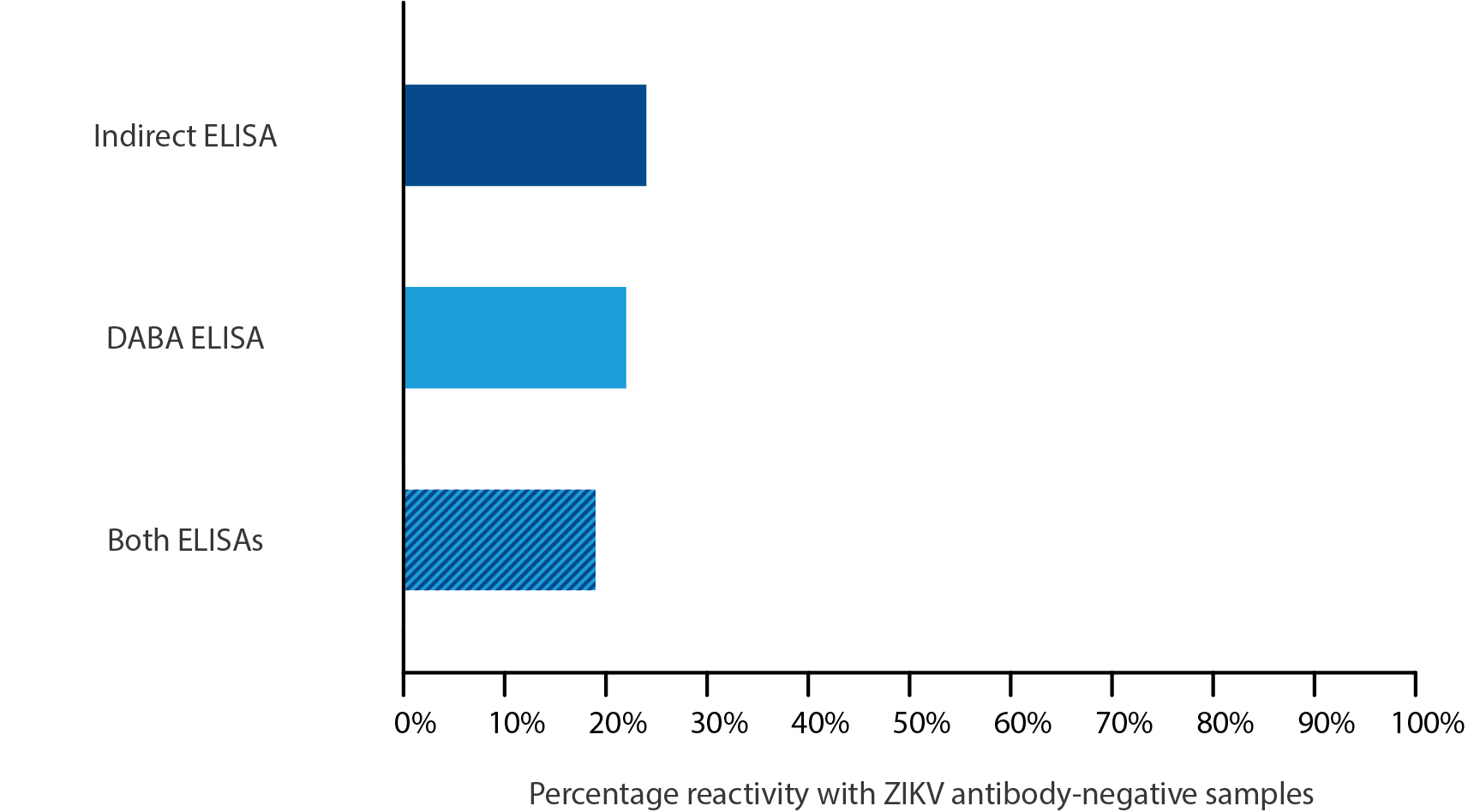
Firstly, assay cross-reactivity was tested using 147 samples of patient sera, collected prior to the introduction of ZIKV to Brazil (and therefore likely to be from a Dengue infection). Of the 147 pre-ZIKV samples, 24% were reactive with the indirect capture, 22% were reactive with the unblocked DABA and 19% of samples were reactive in both assays.
It is evident that both assays show significant cross reactivity with antibodies against other flaviviruses present in the sera:
Initial Sensitivity
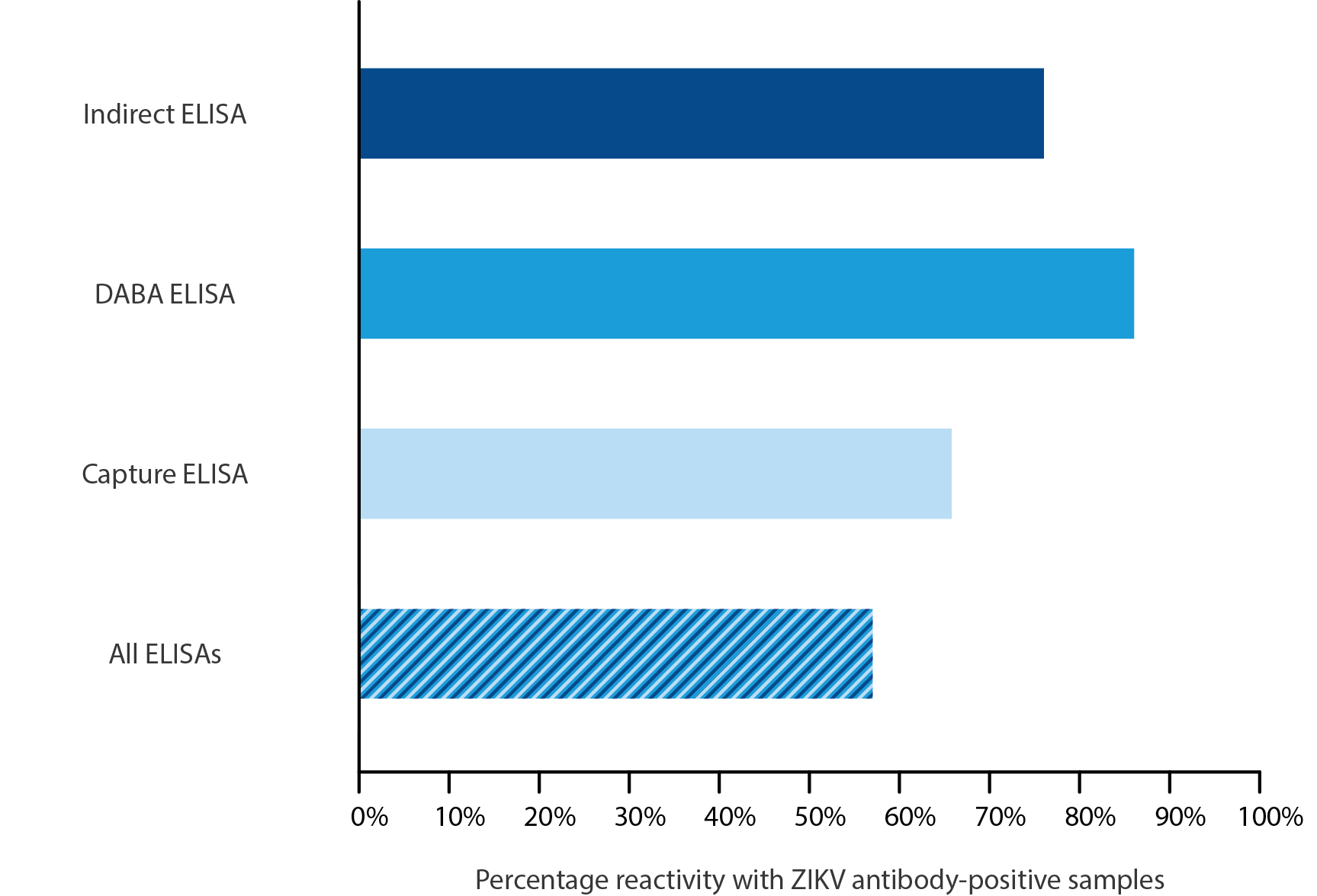
Assay sensitivity was then measured using a panel of 56 patient sera that were undergoing confirmed acute ZIKV infection. Of the 91 sera tested, the indirect capture confirmed infection in 76%, the DABA 86% and the IgG capture 66%. Of the samples tested, 57% were reactive in all three of the assays.
Effects of Quenching
A quenching method was also investigated in the DABA and IgG assays, using our DENV NS1 antigens to bind cross-reacting DENV antibodies and ablate false-positive effects. Our human anti-ZIKV NS1 IgG1 antibody was used as a positive control. A panel of 32 DENV antibody-containing sera were used in a modified conjugate diluent containing the DENV 3 NS1 antigens and an equipotent solution of DENV 1-4 NS1 antigens:
DENV 3 NS1
32 DENV antibody-containing samples were mixed with DENV 3 NS1 and one sample remained reactive with the DABA. All others were successfully quenched.
A subset panel from 95 of the ZIKV-positive patients was then re-tested using DENV 3 NS1, showing equivalent anti-ZIKV reactivity in the majority of samples. 10% of samples in the DABA and 20% in the IgG capture showed a significant reduction of reactivity with blocked conjugates – likely a direct result of reduced false-positives.
DENV 1-4 NS1
20 DENV antibody-containing samples were mixed with equipotent DENV 1-4 NS1 solutions. No samples remained reactive in the DABA and two were reactive in the IgG capture. Samples remained consistently reactive for anti-ZIKV antibodies.
Compared to the single serotype 3 NS1, the mix of equipotent DENV 1-4 NS1 antigens was best at achieving reduced cross-reactivity in both pre-ZIKV and ZIKV-containing sera.
Summary
All three assay designs showed a significant degree of cross-reactivity. However, the use of quenching was shown to be effective at reducing non-specific reactivity in both the DABA and IgG capture formats.
The quenching method used by Tedder and colleagues to absorb out interfering reactivity poses as an effective means of increasing assay specificity, while retaining sensitivity. In particular, the DABA assay is shown to be well-fit for this method as it is a ‘total’ antibody assay that allows for high sensitivity of detection for a range of different anti-ZIKV antibody.
The Native Antigen Company’s proprietary mammalian expression system is used to produce high-quality recombinant ZIKV antigens, with proper glycosylation and folding – much the same as native proteins. These have been used to raise highly specific, well-characterised anti-ZIKV antibodies that exhibit no cross-reactivity with other virus species. We also developed a range of ZIKV immunoassays, including a DABA total antibody ELISA kit designed for immunosurveillance applications. It has shown extremely high specificity in areas of endemic DENV infection, where cross-reactivity has been a significant problem with earlier assays.