In the second of a 2-part series, we discuss how adenoviruses are being developed to treat cancer and some of the hurdles these platforms face from our own immune systems.
Curing cancer is one of the biggest challenges of the 21st century. Our knowledge of cancer’s fundamental mechanisms has greatly improved over the last two decades, revealing huge variability between not only the different types of cancer, but also between patients with the same types of cancer. However, survival rates for the more aggressive cancers have not seen much improvement and treatment is often still crude, with many harmful side effects. Over recent years, the development of new technologies in the field of immuno-oncology have shown promise for a new generation of more effective anti-cancer therapeutics, with CAR-T, CRISPR-Cas9 and nanoparticles-based platforms often making the headlines. An emerging field that also shows great promise is anti-cancer virotherapeutics – the use recombinant viral vectors to selectively infect and destroy cancerous tissues.
Adenoviruses for cancer therapy
From as far back at 1904, a link between viral infection and the improved status of cancer patients was reported in the medical literature, with anecdotal observations of spontaneous halting or regression of tumours [1]. In the 1950s the concept of viral cancer therapy really took off, prompting a flurry of clinical trials that sought to investigate treatments by administering patients with all sorts of nasty viruses. The outcome was predictably bad and patients contracted all manner of diseases, causing ethics committees to pull the plug [2].
The adenoviruses (AdVs) showed some initial promise with more favourable safety profiles, but like the other viruses that were studied, they were not effectively translated into a benefit for the patient [3]. Interest inevitably waned and the studies were lost to the medical literature for some time. Since then, oncolytic virotherapies have gone through boom and bust cycles, reaching feverish peaks of inflated expectations, to troughs of disappointment.
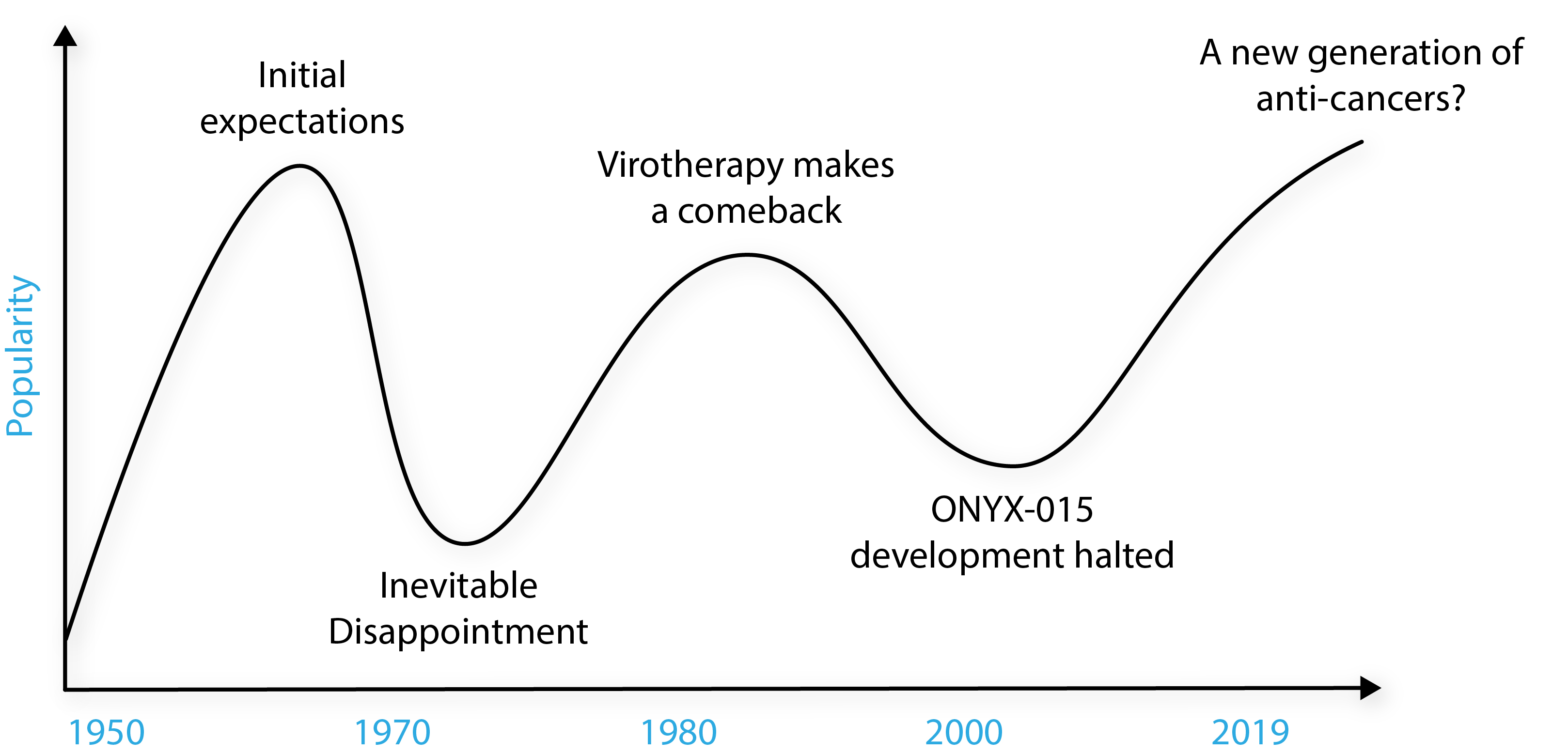
Adapted from Larson et al. [1].
Over recent years, the scientific community has taken a shine to vrotherapies and AdVs in particular, which are used as molecular workhorses for a variety of applications, from basic research, to vectors for the delivery of disease-fighting genes. One of their most promising areas is oncology, where AdVs have evolved into advanced technologies in their own right and have shown a potent ability to mount beneficial immune responses.
Why adenoviruses?
If you want an overview of why AdVs make good vectors for gene therapy you can read our previous blog, but I’ll give you the condensed version: AdVs have some excellent advantages over other viruses for clinical applications, such as genome stability, efficient entry to host nuclei, good gene transduction and no integration with host genomes. The ability of AdVs to purify to high titres and take large DNA inserts also makes them favourable over other oncolytic retro- or lentiviruses in lab settings. A feature of AdVs that is especially useful for cancer treatments is their unique ability to infect a broad range of cell types, which is great for delivering genes to the many tissues in which cancer can be found.
How do they work?
The strategies employed with recombinant oncolytic viruses are numerous and multi-pronged, often leveraging various overlapping mechanisms of the immune system to identify and kill cancerous cells. The basis of these therapies relies on the inherent ability of AdVs to infect, replicate and lyse membranes. However, AdVs (and viruses in general) have shown a limited ability to eliminate tumours solely by this means, which has led to the use of transgenes with adjuvant-like properties that stimulate the immune response and enhance their efficacy. Mechanisms of transgene function are diverse and far-reaching, but most commonly cause anti-tumour effects by sending the immune system’s alarm bells ringing to mount a coordinated attack against a malignant tissue. A new generation of so-called ‘suicide’ vectors is also being developed which deliver genes for cytotoxic prodrugs under the control of specific tumour promoters [4]. The opportunities for therapeutic intervention are diverse and utilise a spectrum of overlapping and interacting mechanisms, often blurring the line between an anti-tumour drug and immune adjuvant. Unlike other therapeutic vectors, oncolytic viruses also need to replicate and proliferate through a tissue to effectively halt or destroy the tumour (which they naturally excel at), but must do so without harming healthy tissues. And so, viruses are often engineered to be tumour-selective by the deletion of the wild-type genes that are essential for replication, but are complemented in cancer cells with an aberrant cell cycle, DNA-repair, or cell death mechanism [5]. If successful, vectors will only replicate in tumour cells – and will do so at an unprecedented rate as our immune systems tend to turn a blind eye to the goings-on of a tumour’s microenvironment.
Some typical mechanisms are illustrated below:
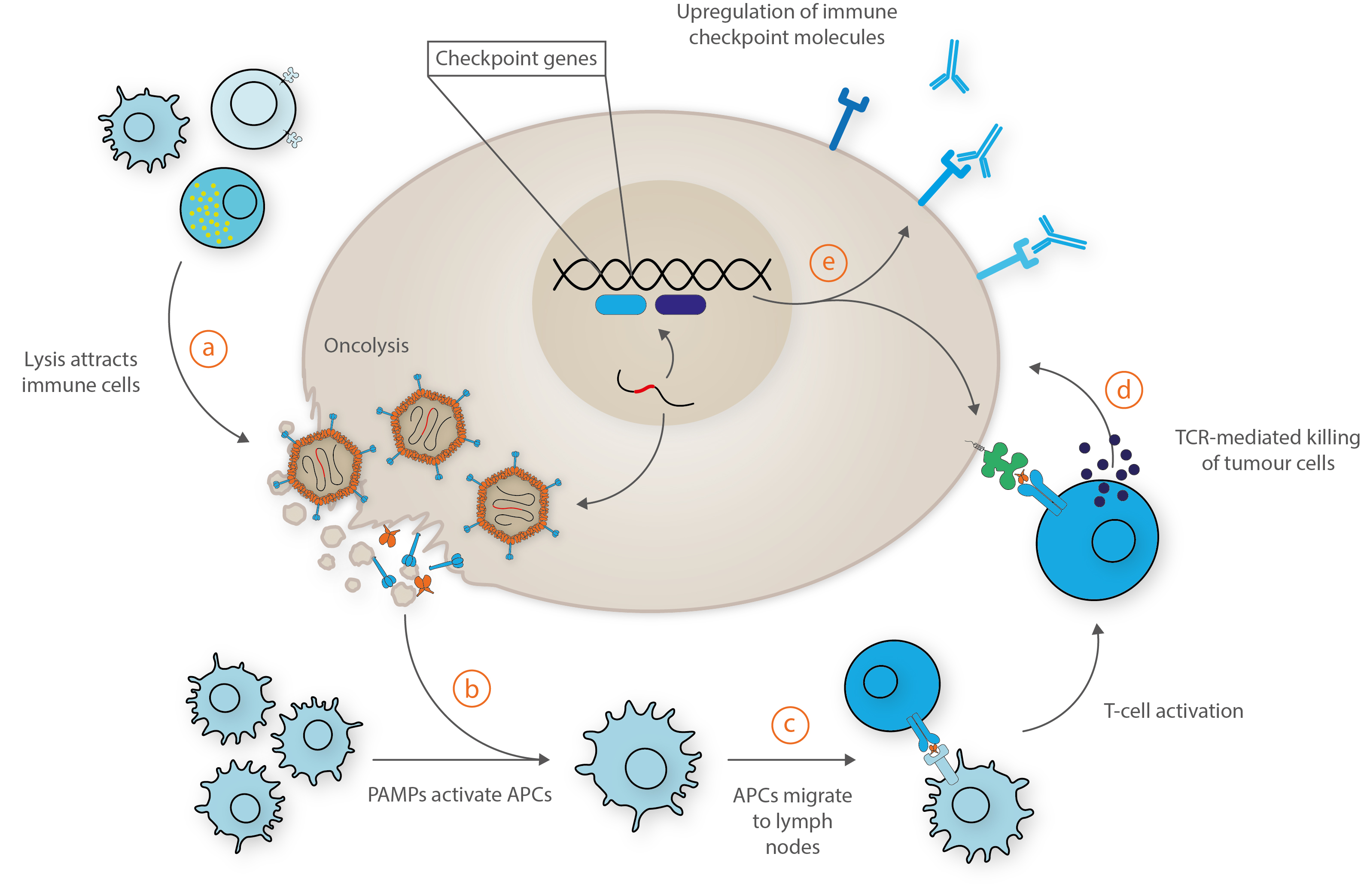
Adapted from Bommareddy et al. [6].
a) Upon administration, viruses infect, replicate and lyse the tumour cells, resulting in the release of soluble tumour antigens that attracts immune cells. b) The released athogen-associated molecular patterns activate antigen-presenting cells (APCs). c) Activated APCs migrate to the lymph nodes, bolstered by viral-mediated release of interferons and chemokines to recruit tumour-specific CD8+ T-cells. d) These cytotoxic T-cells recognize and kill tumour cells via release of granzymes and perforins. e) Transgenes and interferons may also increase the expression of immune checkpoint molecules by the tumour cell to promote immune recognition and by programmed or CD8+-mediated cell death.
Sounds fairly straightforward right?
Not quite. As our immune systems have evolved to better protect us from more complex threats, they have produced more elaborate mechanisms to detect and destroy foreign material. Vectors must therefore stealthily slip past a range of host detection systems to deliver their payload while keeping off-target immune responses to a minimum.
Room for improvement?
Sequestration
When AdVs are administered they are often sequestrated in the liver and spleen, where resident macrophages can trap circulating viruses to induce inflammatory responses. This has the effect of negating the vector’s therapeutic potential and can cause significant hepatotoxicity [7]. To remedy this, AdV capsid proteins can be chemically engineered through genetic substitution of protein domains with other AdV serotypes. This can alter AdV tropisms to modulate the time spent circulating in the blood and (hopefully) reduce unwanted immunogenic effects [8].
Issues with sequestration are reflected by the fact that most oncolytic AdV have been designed with intracellular or paracrine anti-tumour activity for a small set of localised cancers that have limited their systemic use [9]. To treat metastasizing cancers, however, vectors will need to move unhindered through the circulatory system by overcoming physical and immunological barriers to reach far reaching tissues.
Off-target immunogenicity
A drawback of using adenoviruses in gene therapy is that they induce several off-target immune signalling pathways. Inside the host cell, AdVs can be recognised by various intracellular sensors, such as RLRs, TLRs, NLRs and DNA-sensing proteins [10]. These, in turn secrete proinflammatory cytokines and lead to robust downstream responses that attempt to clear the virus. While these pathways can often be useful in killing tumour cells, immune reactions are not always specific and can cause off-target toxic effects. A thorough understanding of an AdV vector’s immune interactions is therefore needed to ensure it is sufficiently immunoevasive, yet effective.
Off-target tropisms
Adenoviruses have the ability to infect a broad range of tissues, and while this is very useful for a disease like cancer, it can be a double-edged sword. AdV Fiber proteins (the pointy bits that stick out from the capsid) are able to bind to various receptors present in many different tissues to mediate host cell entry – potentially causing off-target toxicities. AdVs must therefore undergo extensive refinement of their natural tropisms to tailor them tumour-selective and maximise therapeutic efficacy. Once their natural tropisms have been ablated, tissue-specific tropisms must then be introduced that selectively target the right tissue. Tropisms are often achieved through the attachment of targeting ligands, polymer coating and multi-specific adaptor molecules [11]. However, this is no easy feat as common AdV receptors (e.g. Coxsackie) are commonly down-regulated by cancer cells [12] and the availability of cancer-specific ligands is by no means large.
How would virotherapies work with existing treatments?
Though the goal of a ‘cure for cancer’ remains a driving force for funding and research, it is unlikely that virotherapies will unlikely offer a ‘one size fits all’ approach, and will be used in unison with conventional treatments. Individualised treatments will be needed to develop a wide range of therapeutic options that can be used in combination with existing treatments to create the right ‘cocktail’ for each and every cancer. Indeed, there has been a marked interest in using oncolytic AdVs in combinatorial treatments with existing immuno-, chemo- and radiotherapies to boost their efficacy and they have shown promise with synergistic modes of action and some clinically-meaningful results [13].
Enadvenotucirev
An examplary therapy is Enadenotucirev (EnAd) – an A11/Ad3 oncolytic adenovirus platform developed by PsiOxus Therapeutics in Oxford. The vector has a dual mechanism to selectively infect, replicate in and kill tumour cells, as well as stimulating anti-tumour host immune responses. EnAd was developed through so-called directed evolution, whereby different virus strains are passaged in cancer cells and those with the most favourable anti-tumour effects are selected for. This process has allowed for the screening of many different vectors before further adaptation for clinical use and thanks to its design strategy, EnAd can also be administered intravenously to gain access to both primary and metastasising epithelial tumours [14]. The virus is being tested in several clinical trials in combination therapies with drugs such as Keytruda Opdivo and Taxol, which have so far shown good safety levels and positive therapeutic effects [15].
What next?
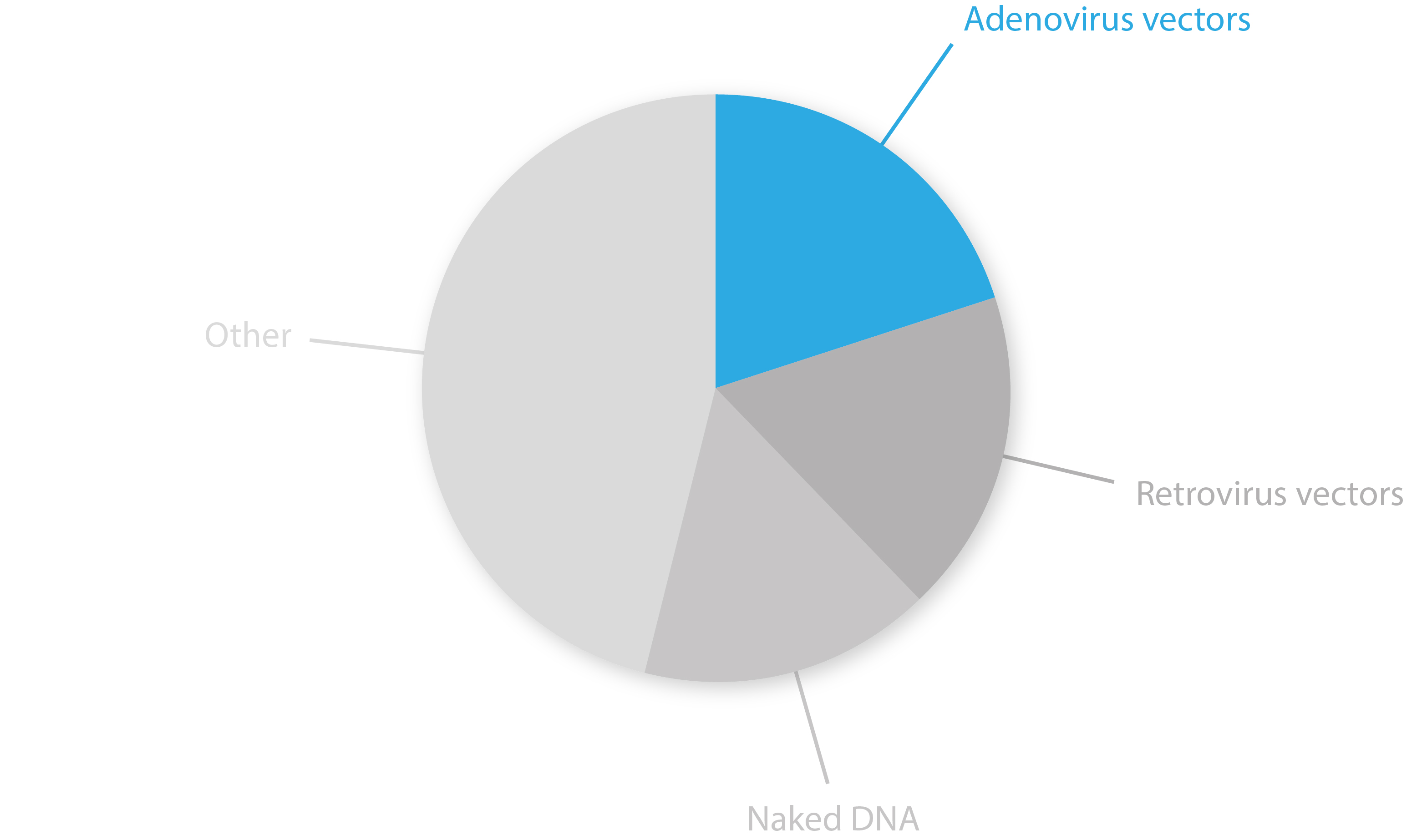
AdV vectors have been through a tumultuous ride of ups and downs over recent decades, but have now stepped into the limelight – accounting for 20% of all gene therapy trials last year [16]. Clinical trials have demonstrated many oncolytic AdVs to be feasible and safe for cancer treatment. However, many experimental vectors have yet to reach adequate efficacies for clinical use as a deeper understanding of their immunomodulatory effects and modes of action are still needed. Further refinement of existing vector platforms, new means of ‘arming’ viruses, and the use of new technologies such as RNAi, polymer-based hybrids and membrane nano-vesicles will likely yield some major breakthroughs in the field over coming years [17, 18].
References
1. https://www.ncbi.nlm.nih.gov/pubmed/26280277
2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4652981/
3. https://www.ncbi.nlm.nih.gov/pubmed/13827367
4. https://www.ncbi.nlm.nih.gov/pubmed/10759680
5. https://www.researchgate.net/publication/281705511_Gene_Therapy_and_Oncolytic_Viruses
6. https://www.nature.com/articles/s41577-018-0014-6
7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC400378/
8. https://www.ncbi.nlm.nih.gov/pubmed/26453806
9. https://www.ncbi.nlm.nih.gov/pubmed/28755290
10. https://www.intechopen.com/books/adenoviruses/adenoviral-vector-based-vaccines-and-gene-therapies-current-status-and-future-prospects
11. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2903974/
12. https://www.ncbi.nlm.nih.gov/pubmed/30905206
13. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3914551/
14. https://psioxus.com/technology-products/enadenotucirev/
15. https://immuno-oncologynews.com/enadenotucirev/
16. http://www.abedia.com/wiley/vectors.php
17. https://www.mdpi.com/2073-4409/7/12/228
18. https://www.ncbi.nlm.nih.gov/pubmed/30964695