In September 2018 The Native Antigen Company and Virology Research Services (VRS) were awarded the Medical Research Council (Proximity to Discovery Award for Knowledge Exchange) to test a large panel of our viral antibodies in immunofluorescence applications. This work was funded through the University College London Translational Research Office and aimed to improve utilisation of the available immunofluorescence resources.
Extraordinary recent advances in the field of microscopy and in antibody production and manufacturing have put immunofluorescence (IF) at the forefront of virology research. IF is widely used for high-throughput screenings and high-resolution imaging, providing relevant and detailed information on the replication and spread of unmodified viruses. The success of IF depends on the specificity and sensitivity of the selected antibodies. However, the limited availability of valid IF protocols and reagents is a major obstacle in viral research, creating a barrier to purchase and leading to under-utilisation of the available resources.
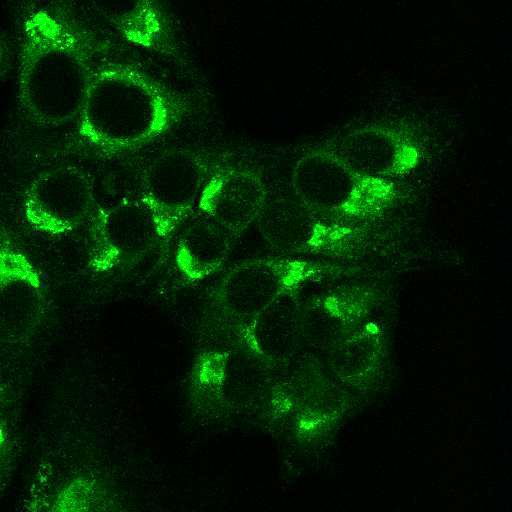
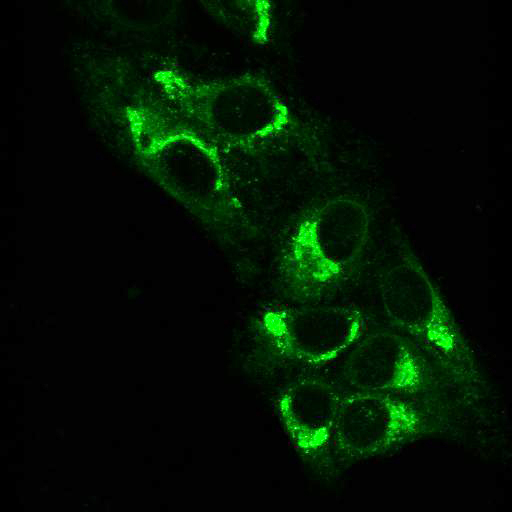
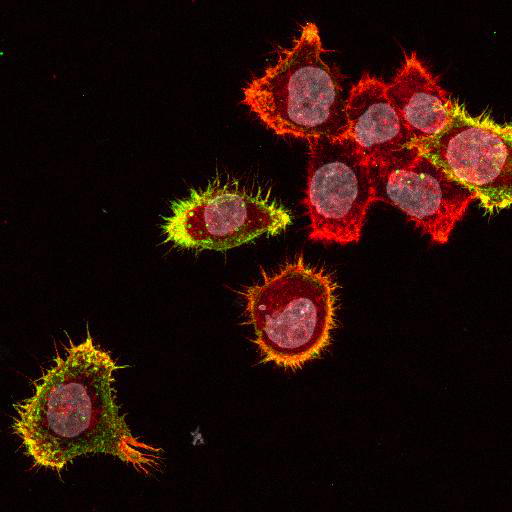
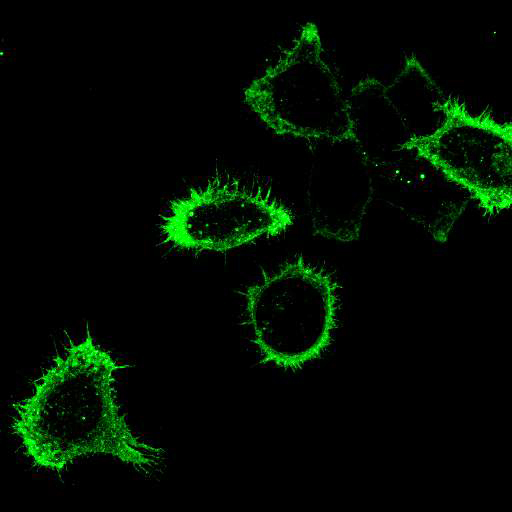
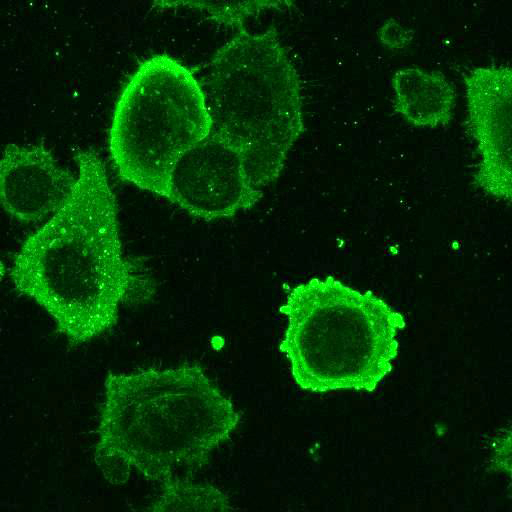
To provide the scientific community with the best possible products for their applications, VRS tested a range of our antibodies against several viruses. In January we published the results of our first series of testing, looking at different serotypes of Dengue and Zika virus. The work featured here comprises the second part of this study, which focused on Cytomegalovirus (CMV), Yellow Fever virus (YFV) and Ebola virus (EBOV).
Procedures
CMV: Human Foreskin Fibroblasts were seeded on coverslips and infected with Cytomegalovirus (CMV) for 96 h at MOI 0.1. Control coverslips consisted of uninfected fibroblasts.
YFV: Vero cells were seeded on coverslips and infected with Yellow Fever 17D virus (YF17D) for 24 h at MOI 2. Control coverslips consisted of uninfected cells.
EBOV: Hela cells were transfected with plasmids expressing Ebola Zaire glycoprotein, nucleoprotein, or VP40, seeded on coverslips and incubated for 48 h. Control coverslips consisted of untransfected cells.
After fixation with 4% PFA, samples were permeabilised with Triton X-100 and stained with our antibodies at a dilution of 1:500 or 1:1000.
High-resolution IF images were taken using an SP5 confocal microscope (Leica).
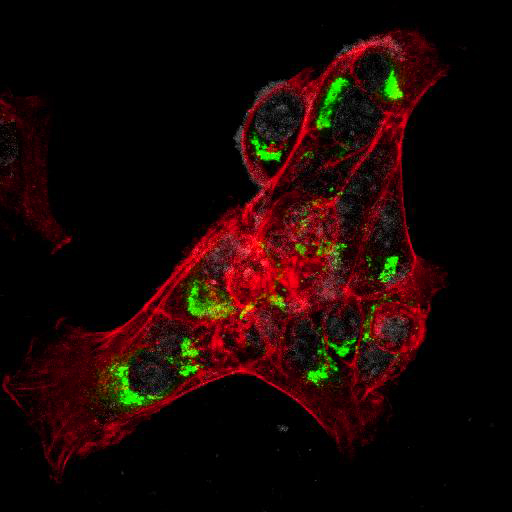
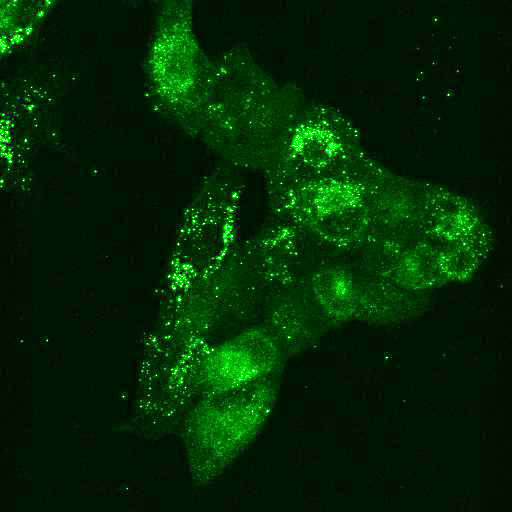
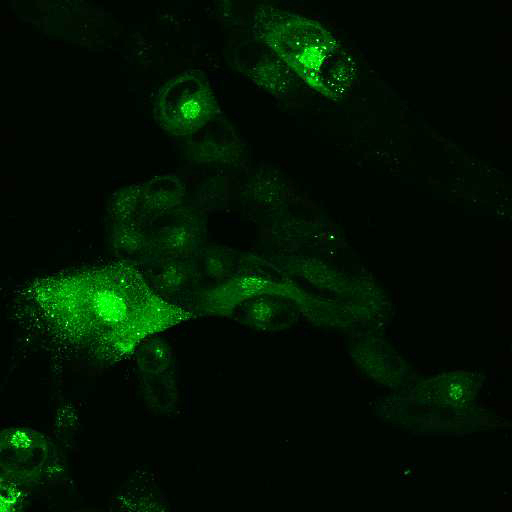
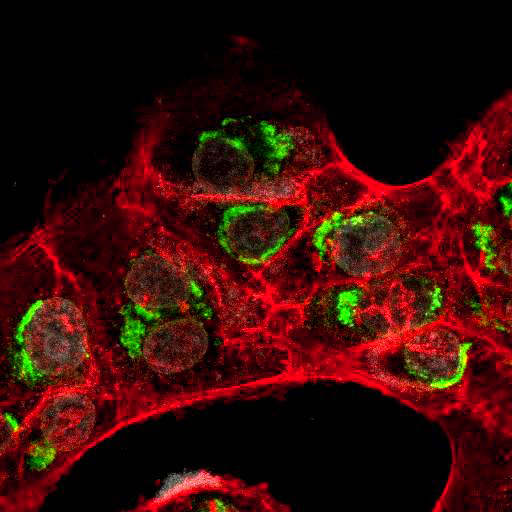
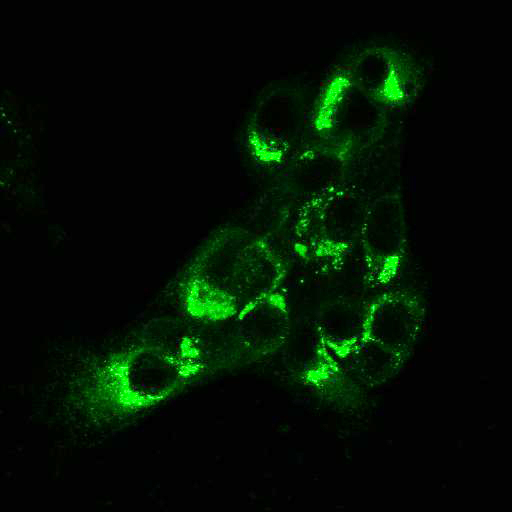
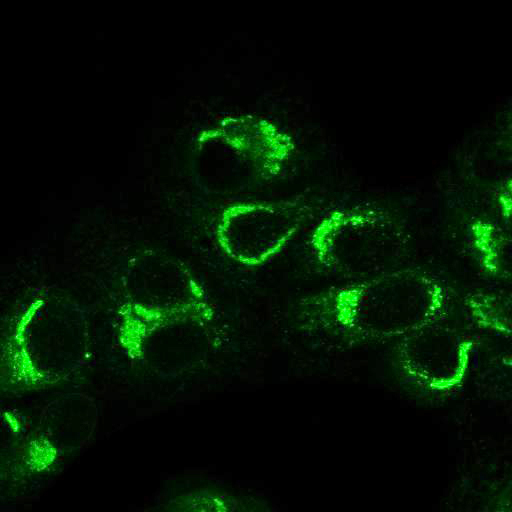
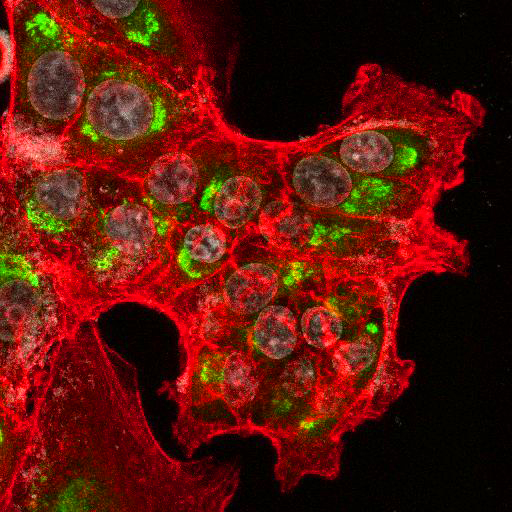
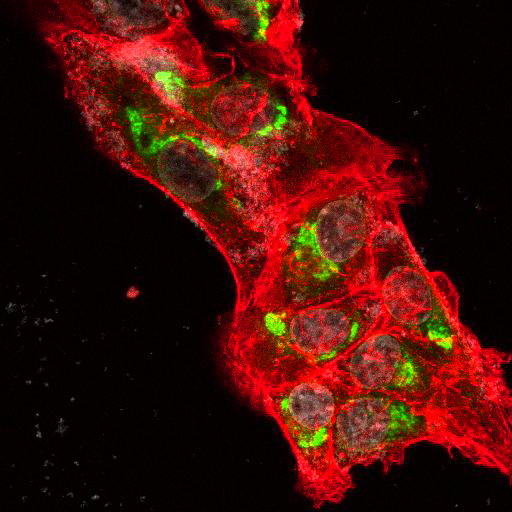
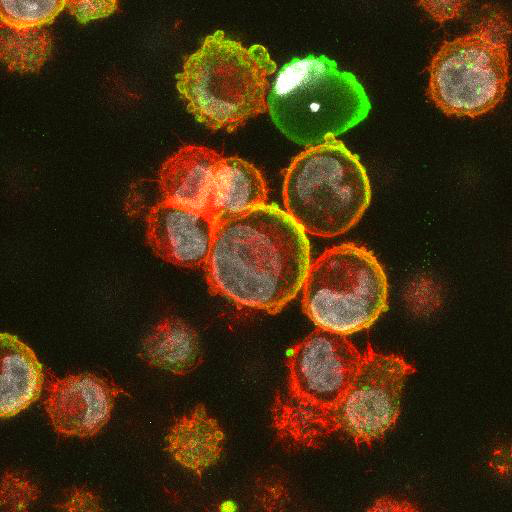
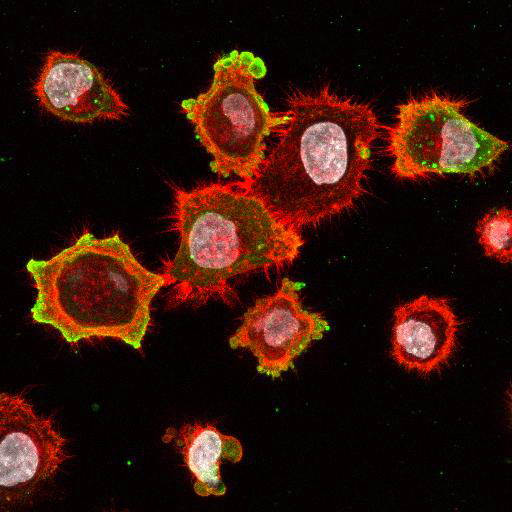
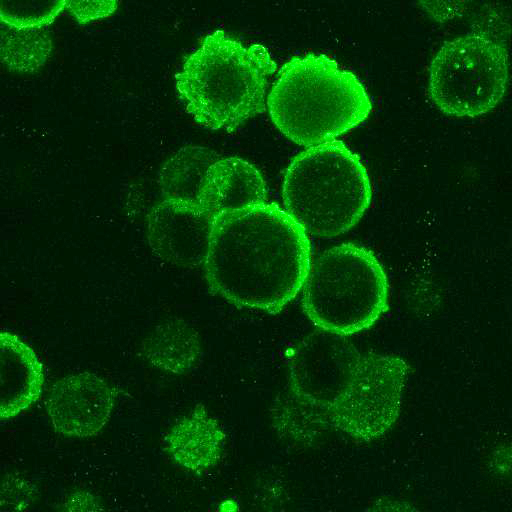
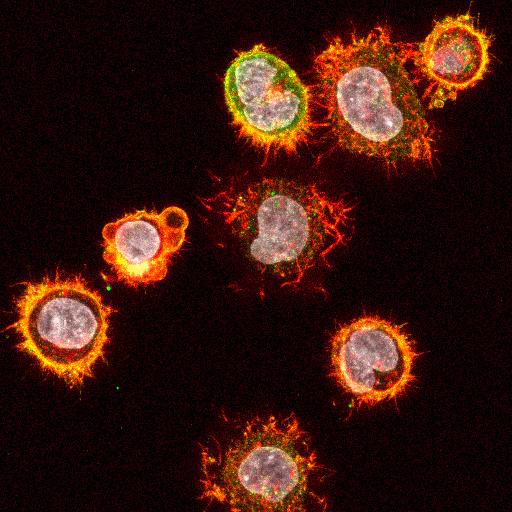
High Resolution Imaging Results
Using a dilution of 1:500 for antibodies against CMV and Ebola proteins or 1:1000 for antibodies against YFV resulted in excellent specificity (negative staining in the no-viral infection/transfection controls) for all tested antibodies. A full protocol for staining can be found here. Examples of the IF results produced by VRS are shown below.
This testing shows that good results in IF applications are highly dependent on the antibodies used and that many NAC antibodies perform well in immunostaining. Such data is extremely valuable in being able to recommend the best reagents to customers for their specific application.
This data is now available on our website for each antibody, to ensure researchers can make better-informed decisions and get the best results at the bench.
Virology Research Services Ltd. is based at UCL and provides virology-focused research services to industry and academia. To find out how they can help with your research, visit virologyresearchservices.com.