The most crucial step for viruses during human infection is recognition and entry into host cells. For this to happen, specific proteins on the virus capsid interact with those on the host cells’ surface, through a ‘lock-and-key’-type mechanism that mediates endocytosis into the host cell.
Human cell surface receptors (CSRs) are therefore being studied as key proteins for the development of novel therapeutics. However, interventions must also take into account that CSRs weren’t designed to mediate viral entry and have their own biological functions for the host, such that blocking the receptor’s function will likely have consequences on host biology. And while there can be clear relationship between a receptor and the binding of a specific virus, in many cases CSRs are more ‘promiscuous’ and are able to bind a range of viruses in a much less specific fashion.
To support further research in this area, we have produced a panel of recombinant human CSRs that are known to mediate virus entry. In many cases, we also provide the viral proteins that bind the corresponding CSR for molecular interaction studies.
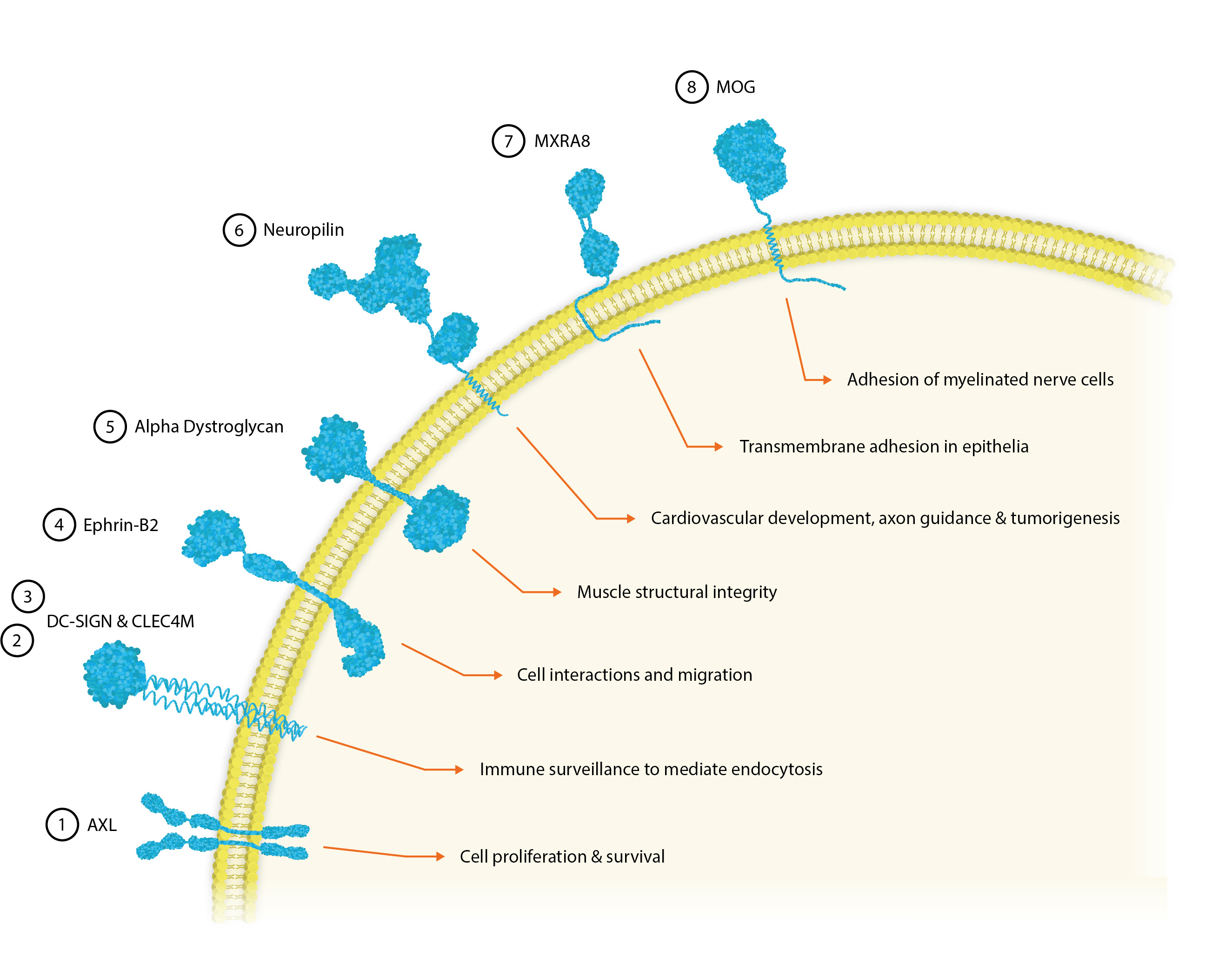
① AXL
Human tyrosine protein kinase receptor UFO is encoded by the axl gene and is expressed in many normal tissues, particularly in bone cells. The AXL CSR is responsible for transducing signals from the extracellular matrix into the cytoplasm by binding GAS6 to facilitate cell proliferation and survival [1]. Therefore, AXL also plays a key role in immune-evasion and drug-resistance in aggressive and metastasizing cancers.
AXL has been shown to significantly enhance infection in a range of viral pathogens, including the Ebola, Influenza A, West Nile, Dengue and Lassa viruses [2].
② DC-SIGN
The human CSR, dendritic cell-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN) can be found on immature dendritic cells and macrophages in peripheral tissues like the placenta and lungs, as well as mature DCs in lymphoid tissue [3]. Here, it shows dual functionality in cell adhesion and the recognition and binding of mannose-type carbohydrates, commonly found on viruses, bacteria and fungi to mediate antigen internalization, processing, and presentation to T-cells.
However, viruses have also evolved to take advantage of DC-SIGN’s antigen-internalizing functionality. Immature dendritic and Langerhans cells are found in the skin and are the first targets for DENV infection, which along with HIV and Hepatitis C, bind the DC-SIGN CSR in the initial stages of infection [4]. Viral antigens that bind DC-SIGN may be potential targets for delivery as vaccines [3] and as DC-SIGN can also be found on DCs and M2-like macrophages in tumour tissue it may show promise as an independent marker for the early diagnosis of cancers [5].
③ CLEC4M
C-Type Lectin Domain Family 4, member M (CLEC4M) is an integral membrane protein that shares 77% amino acid identity with DC-SIGN and is expressed predominantly on endothelial cells of the liver and lymph nodes [6]. Its extracellular region consists of two domains that confer a dual function as a pathogen recognition receptor and cell adhesion receptor through the binding of carbohydrate ligands.
CLEC4M is thought to be involved in peripheral immune surveillance in the liver, mediating endocytosis of pathogens and appears to selectively recognize a variety of surface glycoproteins that contain mannose N-linked oligosaccharides, including HIV1 and 2, SIV, Ebola virus, HCV and SARS-CoV.
④ Ephrin-B2
Ephrin-B2 is a member of the ephrin receptor tyrosine kinase family [7]. Ephrin receptors facilitate many different cell-cell interactions and modulate cell migration in remodelling events – namely in the nervous system. Ephrin-B2 is expressed in most human tissues and promiscuously binds adjacent cells leading to contact-dependent bidirectional signaling.
Ephrin-B2 is also a functional receptor for the Hendra and Nipah viruses, which bind via their G glycoproteins to mediate internalisation [8].
⑤ Alpha-Dystroglycan
Alpha-dystroglycan (DAG) is a glycoprotein receptor for the extracellular matrix proteins, Laminin-2 and Agrin in Schwann cells. It is found in skeletal muscle, where it forms dystroglycan complexes with other proteins to provide structural integrity to muscle tissues [9].
Alpha-dystroglycan also acts as a receptor for the Lassa Fever, Lymphocytic Choriomeningitis and class C new-world arenaviruses [10].
⑥ Neuropilin-2
Neuropilin-1 and 2 are transmembrane glycoproteins that act as non-tyrosine kinase co-receptors. These homologues can both homo- and heteromultimerise with themselves and with semaphorin and VEGF growth factors to facilitate cardiovascular development, axon guidance and tumorigenesis [11].
NRP-2 acts as an entry receptor for furin-processed glycoproteins of the CMV, T-lymphotropic virus-1 and the Epstein-Barr virus [12].
⑦ MXRA8
Matrix Remodelling Associated 8 (MXRA8) is a surface receptor found ubiquitously in human organs and cell lines where it modulates various signalling pathways and mediates cell-cell interactions [15].
MXRA8 is a receptor for multiple arthritogenic alphaviruses, including Chikungunya, Ross River, Mayaro and O’nyong’nyong virus. Alphaviruses cause debilitating arthritis and affect millions in areas where their Aedes mosquito vectors are found. Despite their clinical impact, there are no therapies or vaccines for alphavirus infections. A high-resolution crystal structure of MXRA8 was recently produced and used with cryo-EM CHIKV data to produce the structure of a CHIKV-MXRA8 binding complex, showing that MXRA8 binds the cleft formed by two CHIKV E2-E1 heterodimers [16].
⑧ MOG
Myelin Oligodendrocyte glycoprotein (MOG) is minor component of myelin, found on the outer surfaces of myelin sheaths. It is believed to play an important role in nerve myelination in the central nervous system and to provide structural integrity to the myelin sheath.
In addition to being a dominant target for multiple sclerosis therapeutics, MOG is one of the entry receptors used by the Rubella virus to mediate cell entry [17].
References
1. https://www.uniprot.org/uniprot/P30530
2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4860115
3. https://www.frontiersin.org/articles/10.3389/fimmu.2018.00990/full#B5
4. https://link.springer.com/chapter/10.1007%2F978-1-4614-4433-6_2
5. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0114748
6. https://www.ncbi.nlm.nih.gov/pubmed/11257134
7. https://www.ncbi.nlm.nih.gov/pubmed/15469835
8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1169237
9. https://www.sciencedirect.com/topics/neuroscience/alpha-dystroglycan
10. https://jvi.asm.org/content/79/22/14297
11. http://www.bloodjournal.org/content/108/4/1243?sso-checked=true
12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4705917
13. https://www.uniprot.org/citations/18366072
14. https://www.cell.com/cell/fulltext/S0092-8674(19)30392-7