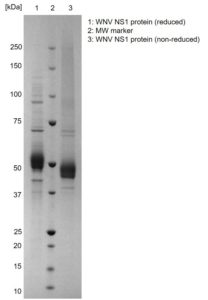
SDS-PAGE: Coomassie-stained SDS-PAGE (Novex 4-12% Bis-Tris gel; Unstained BioRad marker) showing WNV NS1 protein in reducing and non-reducing conditions.
West Nile Virus NS1 Protein
$470.81 – $1,926.57 excl. VAT
Recombinant West Nile Virus NS1 protein produced in mammalian HEK293 human cells, incorporating a C-terminal 6x His-tag.
WEST NILE NS1 PROTEIN
NAC’s West Nile Virus NS1 protein is produced entirely from human cell lines using state-of-the-art expression techniques, the same approach which NAC used to develop its well received DENV NS1 (4 serotypes) and YFV NS1 proteins.
PRODUCT DETAILS – WEST NILE NS1 PROTEIN
- West Nile Virus NS1 protein (Uniprot Q9Q6P4, strain NY99; aa792-114) produced from HEK293 cells.
- Includes a C-term 6His-tag and greater than 95% purity by SDS-PAGE.
- Presented in PBS, pH7.4.
BACKGROUND
The West Nile Virus (WNV) NS1 protein is a native hexameric presentation. The hexamer is believed to be the biologically active form of NS1 involved in key aspects of West Nile Fever pathogenesis (Kaiser et al., 2017). The resultant WNV NS1 protein is purified to a high degree, is in its native folding state, and possesses all post-translational modifications. This advanced approach results in a product which delivers optimal antigenicity by virtue of its human origin
The West Nile Virus NS1 Protein has been manufactured in response to the unmet need for a highly purified, concentrated protein for use in further vaccine development and serological based diagnostic assays. The detection of the NS1 protein offers much higher fidelity across multiple flaviviruses where cross-reactivity can otherwise pose an issue.
REFERENCES
- Kaiser et al. (2017). Virulence determinants of West Nile virus: how can these be used for vaccine design? Future Virol. 12(5):283-295.
****SHIPPING NOTIFICATION: All NS1 products are shipped at ambient temperature. Extensive stability tests have shown no negative effects on antigen performance for 7 days of shipping. Should customers desire shipment on dry ice, this can be performed for an extra fee, please get in contact with us by phone or email.****
Publishing research using our reagents? Please let us know so that we can cite your publication as a reference.
- Nascimento EJM et al. (2018). Development of an anti-dengue NS1 IgG ELISA to evaluate exposure to dengue virus. J Virol Methods. 2018 Mar 19;257:48-57. PMID: 2956751
- Tagliabue G et al (2017). A label-free immunoassay for Flavivirus detection by the Reflective Phantom Interface technology. Biochem Biophys Res Commun. Oct 28;492(4):558-564. PMID: 28501619
- Glasner DR et al (2017). Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog. Nov 9;13(11):e1006673. PMID: 29121099
- Conde JN et al (2016). Inhibition of the Membrane Attack Complex by Dengue Virus NS1 through Interaction with Vitronectin and Terminal Complement Proteins. J Virol. Oct 14;90(21):9570-9581. PMID: 27512066
- Puerta-Guardo H et al (2016). Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. Jul 14;12(7):e1005738. PMID: 27416066
- Beatty PR et al (2015). Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med. Sep 9;7(304):304ra141. PMID: 26355030