In this blog, we discuss the need for a CMV vaccine, the current vaccine strategies that are in development, and introduce our range of CMV antigens and antibodies.
Cytomegalovirus
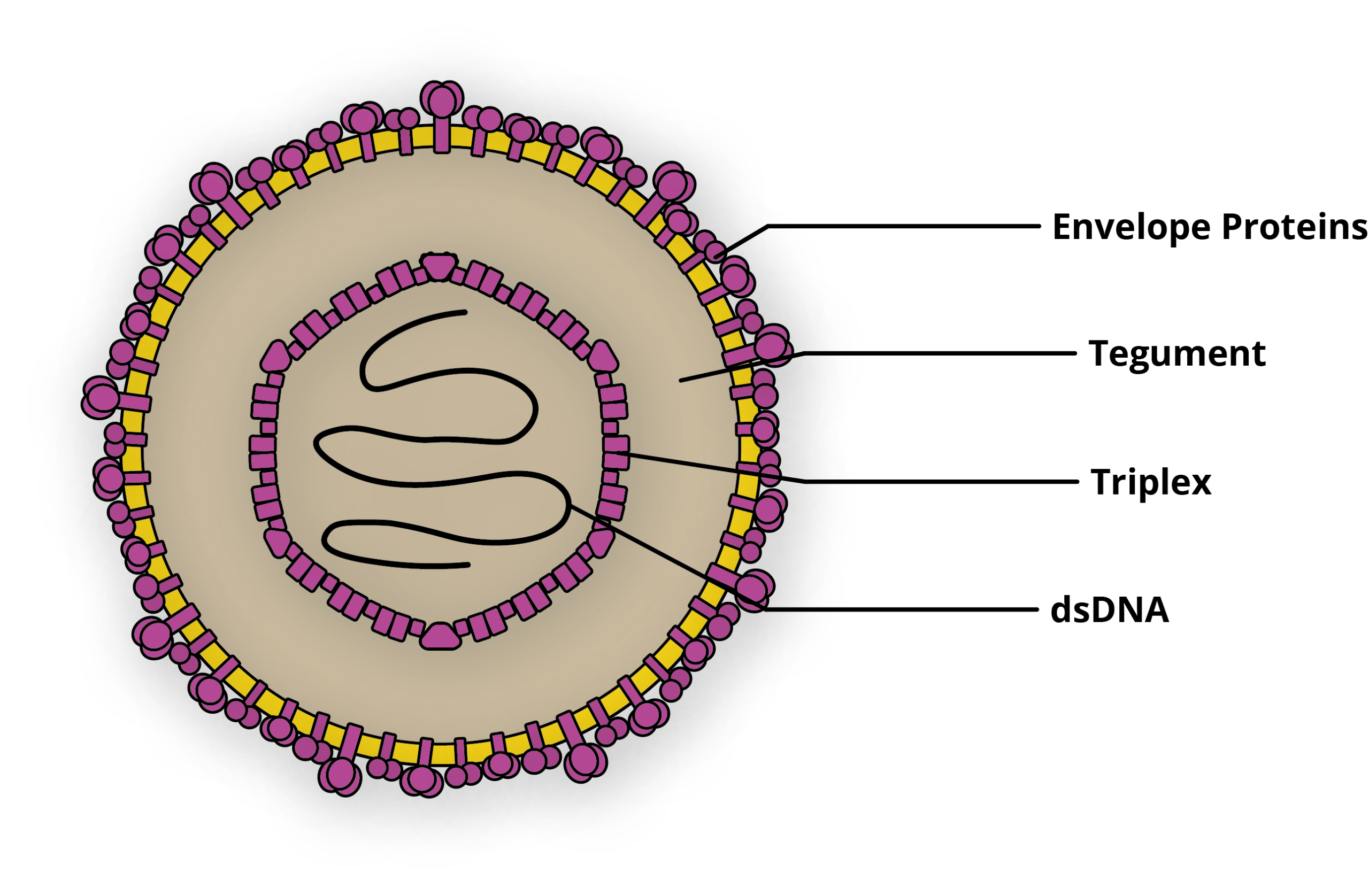
The human Cytomegalovirus (CMV) is an enveloped, icosahedral 150-200nm pleomorphic virus belonging to the Herpesviridae family. CMV’s 236kb genome is comprised of linear, double-stranded DNA, consisting of two unique regions, that are each flanked by inverted repeats [1]. Thought to be one of the most ubiquitous human pathogens, CMV infects virtually 100% of people in low- and middle-income countries [2]. The majority of these infections are asymptomatic, yet CMV causes severe disease in a significant number of pregnant women and organ transplant recipients every year. Like other herpes viruses, CMV can also establish latency after initial infection, before reactivating, and is the most common AIDS related secondary infection as a result [3].
Pregnancy
One of the major populations at risk of CMV infection are pregnant mothers, who have a 30% to 40% chance of vertically transmitting CMV to the fetus [4]. While relatively rare, 1 in every 150 infants are congenitally-infected with CMV (cCMV), making the disease a significant clinical burden [5]. cCMV can result in debilitating sequelae that includes rash, hepatomegaly (enlarged liver), splenomegaly (enlarged spleen), jaundice, microcephaly (small head and brain), cognitive impairment and seizures [6]. cCMV is the most common cause of congenital infections in the developed world and is the largest infectious cause of brain damage and sensorineural hearing loss worldwide [7]. The economic impact is also substantial, estimated to cost $1.9 billion per year in the USA alone [8].
Transplants
CMV is also one of the main agents responsible for infectious complications after organ transplantation [9]. Post-transplant CMV (PT-CMV) occurs due to transmission of the virus from a transplanted organ, which reactivates a latent infection, or occurs after a primary infection in seronegative transplant patients. In immunocompetent hosts, an initial infection is generally asymptomatic, but may present as an non-specific, febrile syndrome. In more rare cases, CMV infection causes a systemic syndrome that affects many organs and includes pneumonia, disease of the gastrointestinal tract, hepatitis and encephalitis [9]. As a result, CMV has a significant impact on the morbidity and mortality of transplant patients, and is a significant drain on diagnostic and therapeutic resources [9]. An emerging body of literature also suggests that CMV is implicated in inflammatory and autoimmune diseases, and may impact the prognosis of burn victims [8].
Vaccine Development Strategies & Candidates
In spite of CMV’s substantial clinical burden, there are no effective countermeasures to prevent infection. Prophylactic antiviral therapies have been invaluable in the management of CMV infection, but are limited by toxicity and the continual emergence of resistance. As such, antiviral toxicity precludes use for cCMV, leaving pregnant women with no protection, other than basic screening. In light of these limitations, a safe and effective vaccine to reduce the incidence and the severity of CMV infection has become a major public health priority worldwide [10].
CMV research took off in the 1970s, when a landmark review described CMV’s impact on the health on infants and transplant recipients for the first time [11]. Since then, research in the field has grown immensely and significant funding and prioritisation have been made to develop a vaccine [12]. Here we discuss some of the major vaccine strategies that are currently being developed:
Attenuated Virus
Potentially, one of the most effective vaccine strategies would be to use attenuated CMV, as it would mimic a natural infection [13]. This spurred some early attempts to develop a vaccine that focused on live attenuated viruses which had been thoroughly passaged in tissue culture. However, it became apparent that attenuated CMV struggled to elicit humoral responses long-term, and risked establishing viral latency [14, 10]. This predisposed recipients to reactivation and complications in later life, making for a high-risk strategy with no clear reward. With this in mind, a new generation of transgenic disabled infectious single cycle (DISC) strains were developed in the 1990s in hope of providing a safer, more effective alternative. DISC strains of CMV are based on UL85 (GP85) mutants, in addition to other selective gene deletions [15]. As DISCs only express a limited subset of the viral proteome, they can be propagated under controlled conditions that allow viral replication in vitro, but are replication incompetent in vivo, and thus prevent risk of latent infection [16].
V160 is an attenuated DISC strain developed by Merck, that is currently undergoing phase I clinical trials in seronegative and seropositive subjects [17]. V160 has a restored wild-type pentamer complex sequence which contains a frameshift mutation in the UL131 sequence. This mutation was repaired in E. coli by a single residue deletion to restore epithelial and endothelial cell tropism and allow proper processing and expression of the pentamer complex [18]. V160 is also modified so that the CMV proteins, IE1/IE2 and UL51 are expressed in fusion with a rapamycin-binding protein [18]. Since UL51 and IE1/2 are essential for replication, V160 is therefore able to propagate in ARPE-19 cells only in the presence of the stabilising ligand, Shield-1. As this synthetic ligand is not found naturally, the fusion protein is rapidly degraded and viral replication is inhibited in immunised subjects, ensuring maximal vaccine safety.
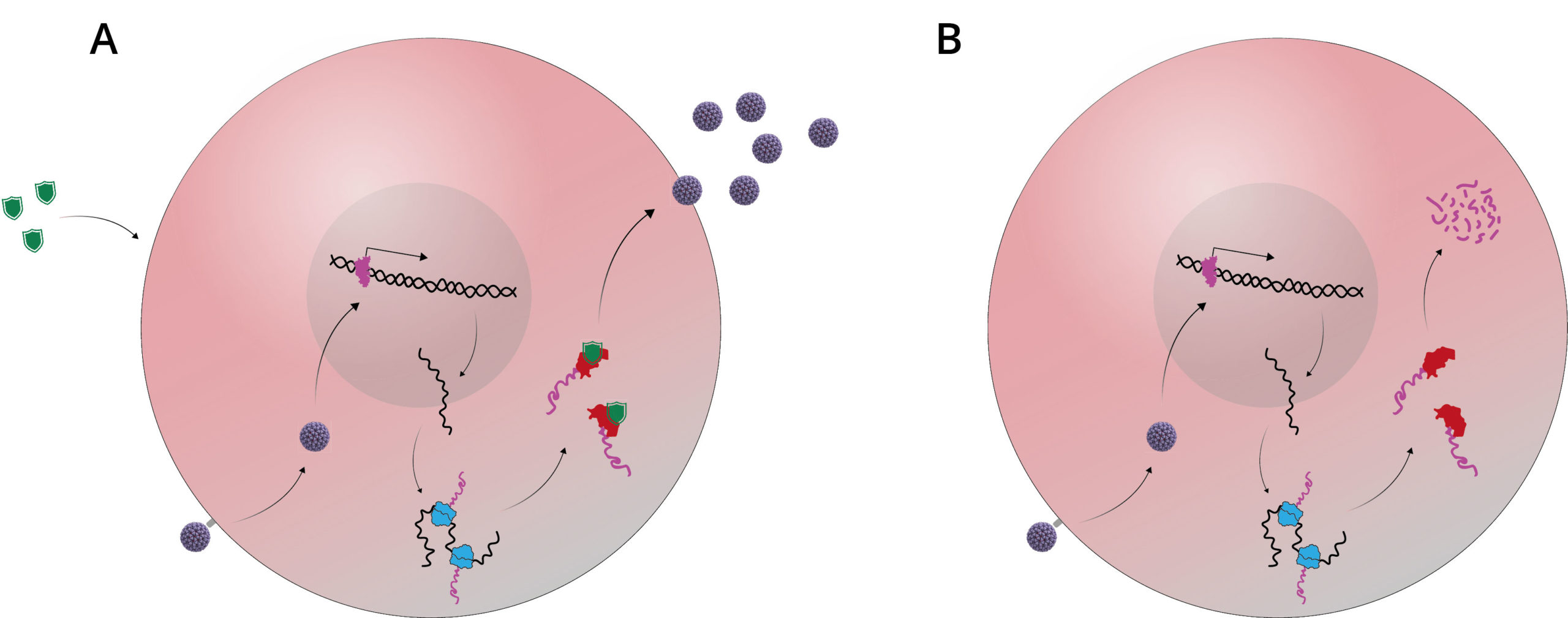
A) In vitro, the Shield-1 ligand is added to cell culture as it is transfected with V160. This stabilises the replication complex and allows proliferation of the attenuated virus; B) In vivo, no Shield-1 ligand is present, which causes destabilisation of the replication complex and does not allow viral assembly.
DNA Vaccines
In contrast to traditional vaccines, DNA vaccines consist of genetically engineered DNA, injected into target cells to produce target antigens ‘on site’. DNA vaccines advanced significantly in recent years and offer a range of potential benefits, including, immunospecificity, safety and cost [19].
ASP0113 is a bivalent CMV DNA vaccine, consisting of two plasmids (VCL-6368) and (VCL-6365), developed by Vical Corporation for haematopoietic cell transplant (HCT) recipients [8]. ASP0113’s added formulation creates self-assembling nanoparticles with defined particle sizes, surface charges and stability. The phase I clinical trial showed no serious adverse events (SAE) [20]. Seronegative subjects elicited gB- and pp65-specific T cell responses and gB-specific antibody responses, whereas seropositive subjects only elicited pp65-specific T cell responses. A subsequent phase II trial continued to show no SAEs, as well as significant reductions in CMV viraemia following vaccination [21]. However, recent phase III trials were reported to be unsuccessful in meeting primary endpoints, as ASP0113 recipients didn’t show significant improvements in overall survival or reduction CMV organ disease [22].
Virus-Like Particles
Several lines of evidence suggest that gB is a major target for neutralizing antibodies (nAb) [23]. However, gB-based vaccines have conferred only short-lived immunity, and have struggled to adequately protect epithelial cells [24]. To overcome this, VBI Vaccines have developed a virus-like particle vaccine (VBI-1501A) to present a form of gB with an altered confirmation that can elicit greater nAb titres [23]. This is achieved by co-expressing transmembrane and cytoplasmic domains of the vesicular stomatitis virus (VSV) G protein, which alter the gB epitopes for nAb recognition. As a result, these gB proteins have been shown to induce more potent nAb responses with greater epithelial cell neutralising activity. A recent phase I study of 128 participants who were administered VBI-1501A showed no SAEs, with most side effects being generally mild [25]. In regards to efficacy, fibroblast cell nAbs were seen in 100% of 2.0µg dose recipients and epithelial cell nAbs in 31%.
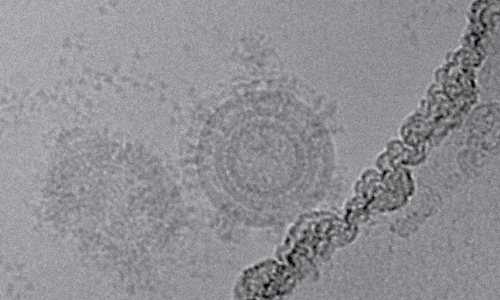
Transmission electron micrograph of VBI-1501A [26].
Subunit Vaccines
Subunit approaches to develop a CMV vaccine have arguably been the most successful to date [18]. In particular, several clinical trials using recombinant CMV gB within a oil/water emulsion with a microfluidised adjuvant have been completed with encouraging results [27, 28]. gB vaccines developed by NIAID, Sanofi Pasteur and Robert Pass are expressed with truncations and are formulated with microfluidised adjuvant 59 (MF59). In a phase II clinical trial of postpartum women, gB/MF59 demonstrated 50% efficacy against primary CMV infection in seronegative women vaccinated within 1 year of giving birth, compared to women in the same cohort who received the placebo, and showed acceptable adverse events [29]. A gB/MF59 trial in solid-organ transplant recipients, showed both a reduction in viraemia, and in the total number of days of antiviral treatment in recipients compared to the placebo group [30].
GSK are developing another gB subunit vaccine (GSK1492903A). GSK1492903A consists of a chimeric gB protein, fused to truncated Herpes Simplex Virus 1 (HSV-1) gD protein, to improve expression and ease of purification. Evaluated in a phase I trial, three-doses administered to CMV-seronegative recipients showed no serious adverse events and robust antibody responses, including neutralising antibodies [31].
Peptide Vaccines
The CMV T-cell response is largely directed against phosphoprotein pp65 – CMV’s only tegument protein [32]. pp65 is recognised by the human leukocyte antigen (HLA) system, which is responsible for encoding the major histocompatibility complex (MHC) proteins. In particular, three specific pp65 epitopes have been shown to display high binding affinity for the HLA-A*0201 serotype, and have been identified as vaccine targets [33].
CMVPepVax is a peptide vaccine under development by The City of Hope medical centre for use in HCT recipients. CMVPepVax is a pp65 peptide, whose epitope is specific to the HLA A*0201 serotype, and is conjugated with the P2 peptide of tetanus toxin, mixed in formulation with a TLR9 agonist adjuvant prior to administration. Combined, the peptide directs the stimulation of specific CD8+ T-cells against the immunodominant pp65 epitope. A 2017 phase I trial demonstrated safety, immunogenicity, increased survival, a reduced rate CMV reactivation and usage of antivirals [34], and a phase II trial is now scheduled [35].
Future Challenges
While significant advances have been made in regards to our understanding of CMV and its immunogenicity, there remain some challenges to developing an effective vaccine. To protect transplantation recipients from infection, stimulating both humoral and cell-mediated immune responses is essential: Antibodies are necessary to prevent infection and spread of CMV in seronegative individuals, while T-cell responses are needed to suppress CMV reactivation in those that are seropositive [36]. Likewise, a vaccine that can protect both seronegative women from primary CMV infections, and augment the immune response to infection in seropositive individuals will be necessary to provide comprehensive protection. Another major hurdle to developing an effective CMV vaccine has been determining how to practically evaluate efficacy. Congenital CMV is rare at a population level (1 in 150 pregnancies) and occurs in mostly vulnerable patient populations, requiring very large samples in resource-limited populations. Likewise, determining practical endpoints for trials is complex, considering that viral spread from children, prevention of maternal infection, blocking of cross-placental transmission and reduction in congenital infection in infants are all relevant [37].
Cytomegalovirus Reagents from The Native Antigen Company
To support ongoing CMV research, The Native Antigen Company provides a range of antigens and antibodies for vaccine and diagnostic development:
Antigens
The Native Antigen Company have developed a range of CMV antigens, including preparations from native virus and highly purified recombinant proteins, expressed in our proprietary VirtuE mammalian cell expression system. Our recombinant antigens include the two key vaccine candidates, glycoprotein B, with mouse and human Fc tags, and CMV pentamer protein, for which The Native Antigen Company is renowned. We also offer purified whole CMV antigen and CMV cell lysate.
Antibodies
The Native Antigen Company offers a range of fully validated anti-CMV antibodies using our antigen range. This range includes CMV-specific antibodies against glycoprotein B, pentamer protein, glycoprotein H, ICP36 and phosphoproteins 28 and 65.
Our sheep anti-CMV pentamer polyclonal has been raised against mammalian-expressed gH pentameric complex, and has been shown to react with each of the components of the pentamer in Western blotting analysis. Our mouse anti-glycoprotein B monoclonal recognises and binds a neutralising epitope on CMV glycoprotein B and has also shown to be effective at binding in direct ELISA.
References
1) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2885759/#r28
2) https://www.sciencedirect.com/science/article/pii/S0264410X18302883
3) http://grantome.com/grant/NIH/R01-AI098984-04
4) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3046747/
5) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5804742/
6) https://www.ncbi.nlm.nih.gov/pubmed/17542052
7) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3082510/
8) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5075346/
9) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4496754/
10) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5628252/
11) https://www.nejm.org/doi/full/10.1056/NEJM197107222850406
12) https://www.ncbi.nlm.nih.gov/books/NBK233313/
13) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4988156/
14) https://www.ncbi.nlm.nih.gov/pubmed/6329366
15) https://www.caister.com/cmv2v1
16) https://www.ncbi.nlm.nih.gov/pubmed/24681264
17) https://clinicaltrials.gov/ct2/show/NCT03486834
18) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5717185/
19) https://www.sciencedirect.com/science/article/pii/S222116911530366X
20) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2956065/
21) https://www.ncbi.nlm.nih.gov/pubmed/22237175
22) https://www.astellas.com/en/news/10206
23) https://cvi.asm.org/content/21/2/174.long
24) https://www.ncbi.nlm.nih.gov/pubmed/23041121?dopt=Abstract
25) https://idsa.confex.com/idsa/2018/webprogram/Paper74253.html
26) https://www.vbivaccines.com/technology/evlp-platform-overview/
27) https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(11)60136-0/fulltext
28) https://academic.oup.com/jid/article/203/11/1534/863062
29) https://www.nejm.org/doi/full/10.1056/NEJMoa0804749
30) https://linkinghub.elsevier.com/retrieve/pii/S0140673611601360
31) http://www.gsk-clinicalstudyregister.com/study/108890#rs
32) https://jvi.asm.org/content/78/20/10995
33) https://www.jimmunol.org/content/163/10/5512.long
34) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4926626/
35) https://ichgcp.net/clinical-trials-registry/NCT02396134
36) https://www.sciencedirect.com/science/article/pii/S0264410X18302883
37) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6148244/