New SARS-CoV-2 Neutralisation Assay Development Kits
• Delta Variant
• Kerala Variant
• South African Variant
• Brazilian Variant
• UK Variant
• Wild-Type
With all the key reagents required to measure Spike-antibody binding, these easy-to-use, cell-free kits can be used to identify and qualitatively assess the ability of antibodies to neutralise variant RBD-ACE2 interactions. For more information, please see the information below:
Delta Variant (B.1.617.2)
Our SARS-CoV-2 Delta Variant Neutralisation Assay Development Kit is designed for the qualitative assessment of neutralising antibodies to the B.1.617.2 RBD (L452R, T478). In-house data shows that different SARS-CoV-2-positive patient sera is able to variably neutralise binding:
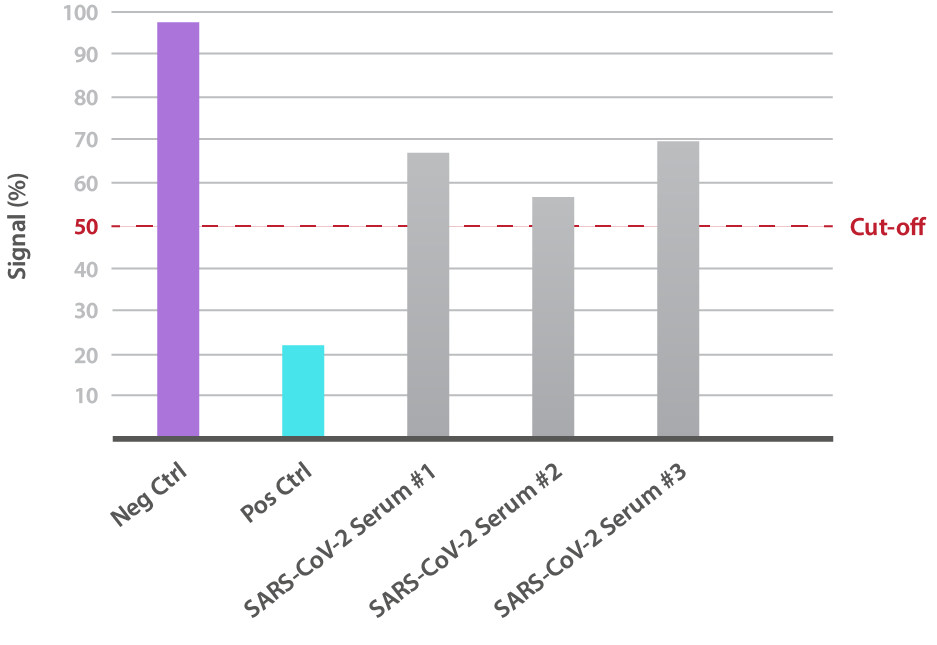
NTRL Assay: Binding rate (B/B0%) for negative samples is ≥ 60%. Binding rate for strongly positive samples is ≤ 50%. Borderline samples, with moderate neutralization, may be assessed with binding rates between 50-60. Negative and positive control antibodies were included. SARS-CoV-2 serum sample #2 is between 50% and 60% and is therefore positive for the presence of neutralizing antibodies. SARS-CoV-2 serum samples #1 and #3 are >60%, indicating no neutralizing capacity.
Kerala Variant (Kappa/B.1.617.1)
Our SARS-CoV-2 Kerala Variant Neutralisation Assay Development Kit is designed for the qualitative assessment of neutralising antibodies to the B.1.617.1 RBD (E484Q, L452R). In-house data shows that different SARS-CoV-2-positive patient sera is able to variably neutralise binding:
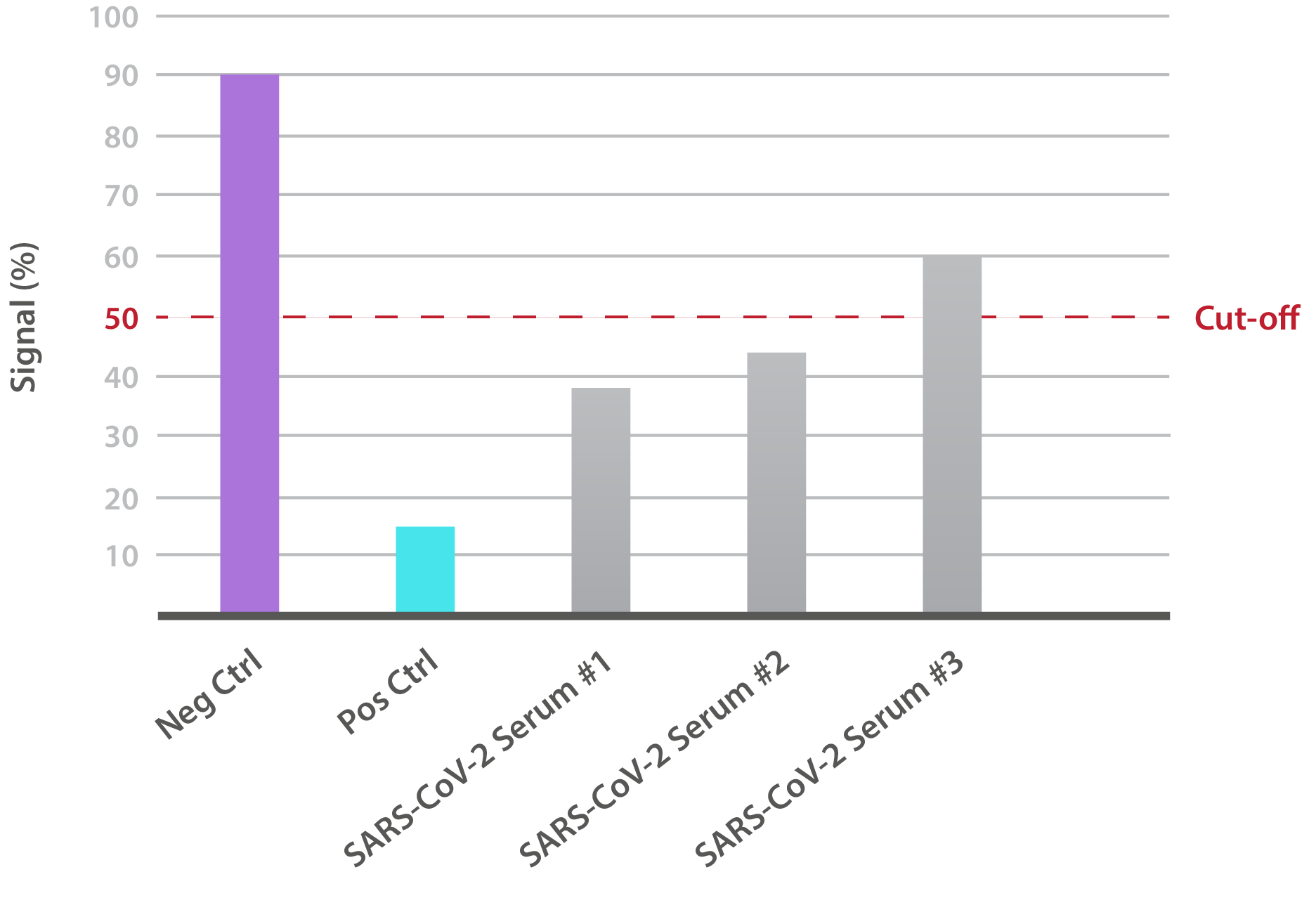
NTRL Assay: Binding rate (B/B0%) for negative samples is ≥ 60%. Binding rate for strongly positive samples is ≤ 50%. Borderline samples, with moderate neutralization, may be assessed with binding rates between 50-60. Negative and positive control antibodies were included. SARS-CoV-2 serum samples #1 and #2 are <50% and therefore positive for the presence of neutralizing antibodies. Serum sample #3 is 60%, indicating no neutralizing capacity.
South African Variant (Beta/B.1.351/501Y.V2)
Our SARS-CoV-2 South African Variant Neutralisation Assay Development Kit is designed for the qualitative assessment of neutralising antibodies to the B.1.351 RBD (K417N, E484K, N501Y). In-house data shows that SARS-CoV-2-positive patient sera is able to neutralise binding:
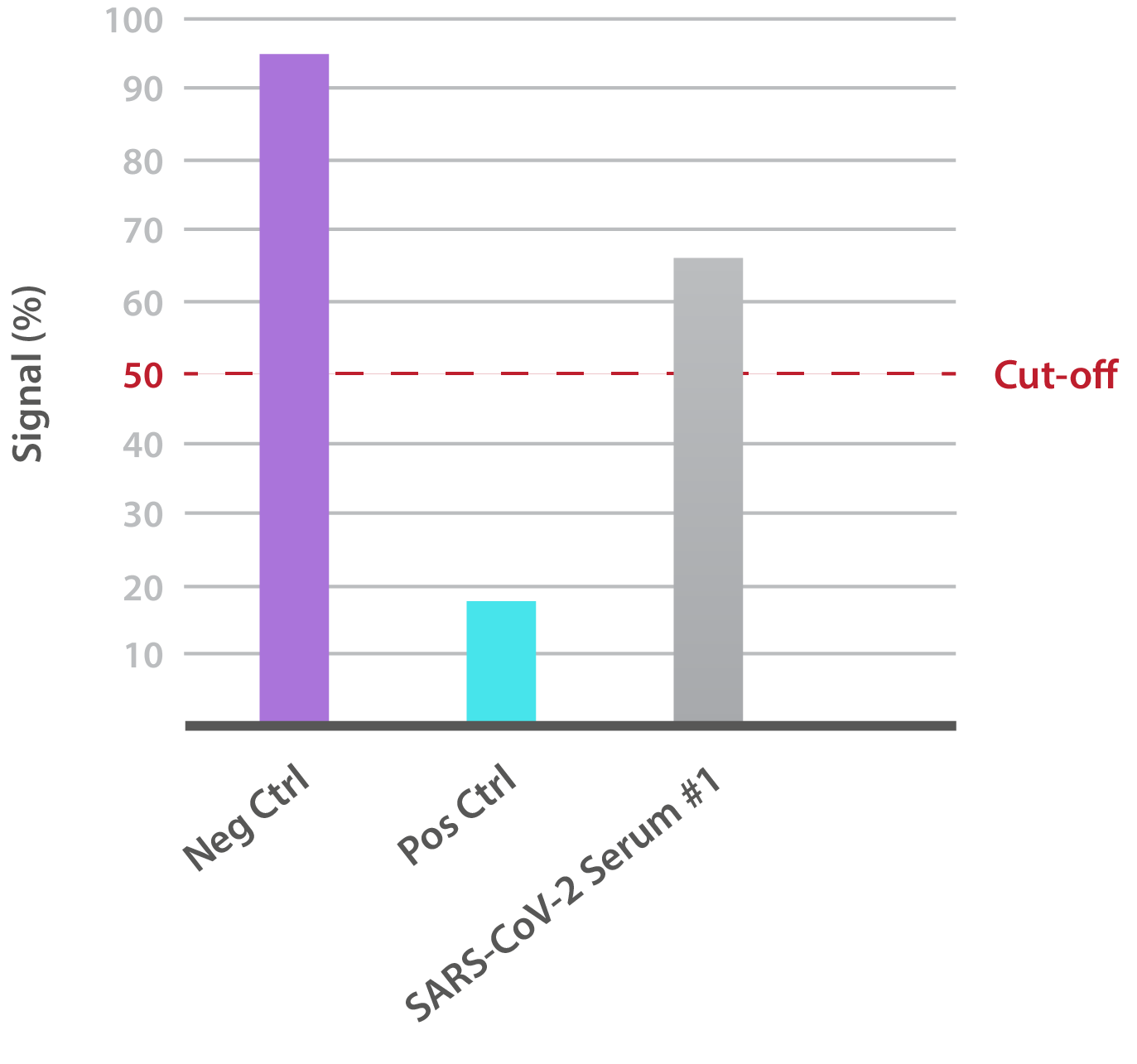
NTRL Assay: Binding rate (B/B0%) for negative samples is ≥ 60%. Binding rate for strongly positive samples is ≤ 50%. Borderline samples, with moderate neutralization, may be assessed with binding rates between 50-60. Negative and positive control antibodies were included. SARS-CoV-2 serum sample #1 is >60%, indicating that neutralizing antibodies are absent.
Brazilian Variant (Gamma/B.1.1.28)
Our SARS-CoV-2 Brazilian Variant Neutralisation Assay Development Kit is designed for the qualitative assessment of neutralising antibodies to the B.1.1.28 RBD (K417T, E484K, N501Y). In-house data shows that SARS-CoV-2-positive patient sera is able to neutralise binding:
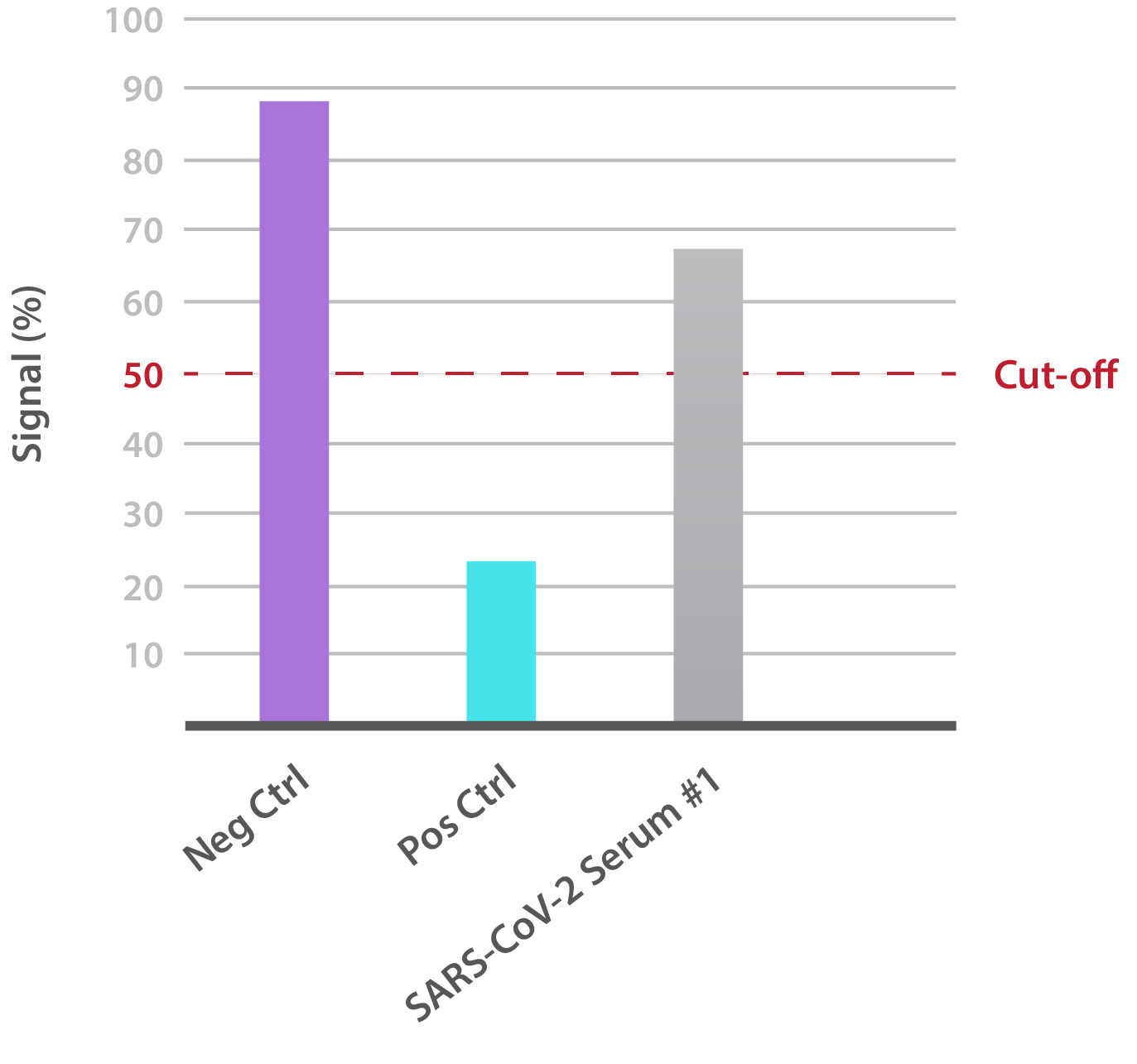
NTRL Assay: Binding rate (B/B0%) for negative samples is ≥ 60%. Binding rate for strongly positive samples is ≤ 50%. Borderline samples, with moderate neutralization, may be assessed with binding rates between 50-60. Negative and positive control antibodies were included. SARS-CoV-2 serum sample #1 is >60%, indicating no neutralizing capacity.
UK Variant (Alpha/B.1.1.7)
Our SARS-CoV-2 UK Variant Neutralisation Assay Development Kit is designed for the qualitative assessment of neutralising antibodies to the N501Y RBD. In-house data shows that SARS-CoV-2-positive patient sera is able to neutralise binding:
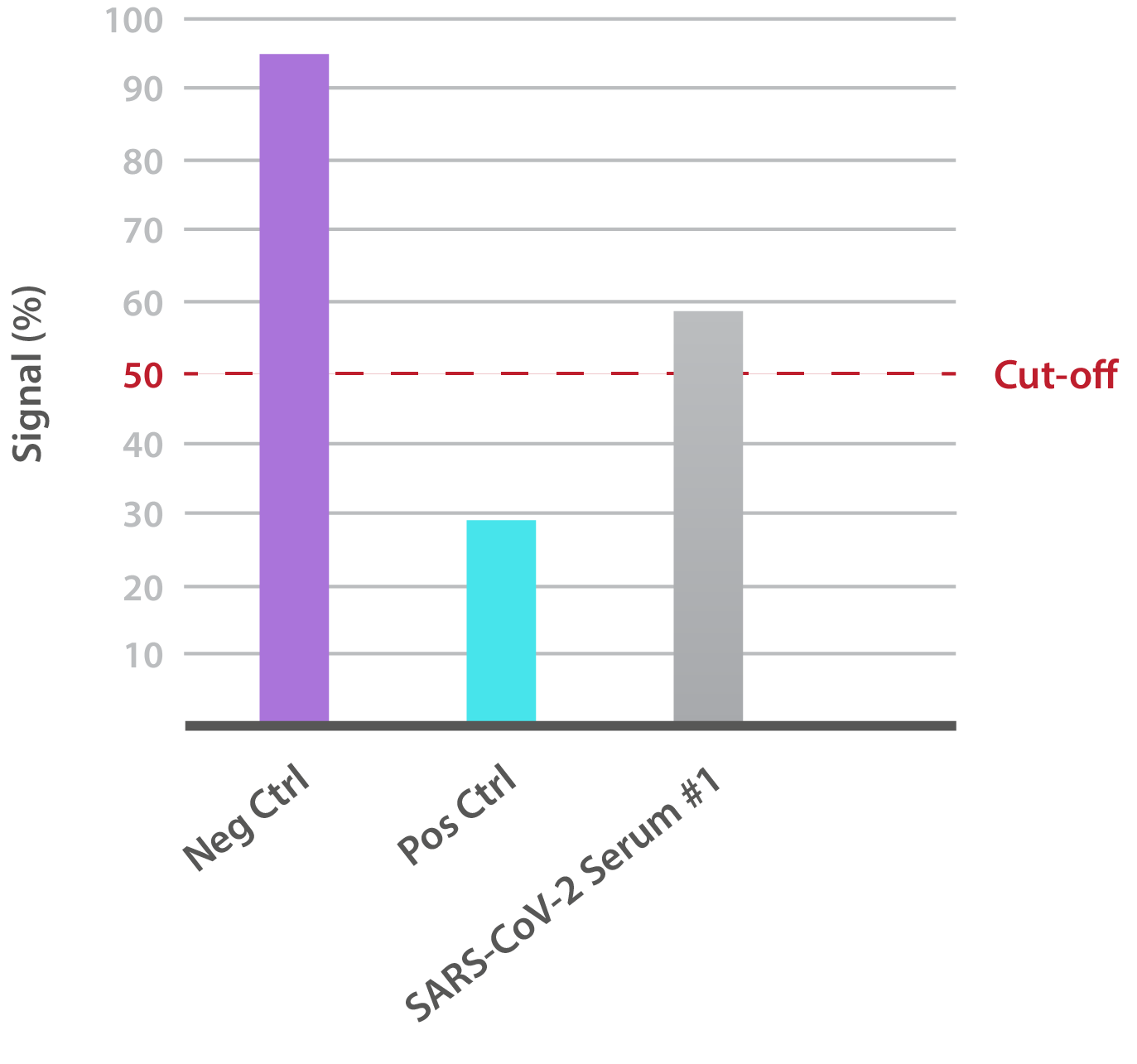
NTRL Assay: Binding rate (B/B0%) for negative samples is ≥ 60%. Binding rate for strongly positive samples is ≤ 50%. Borderline samples, with moderate neutralization, may be assessed with binding rates between 50-60. Negative and positive control antibodies were included. SARS-CoV-2 serum sample #1 is >50% but <60%, indicating moderate neutralizing capacity.
Wild-Type (Wuhan-Hu-1)
Our SARS-CoV-2 Neutralisation Assay Development Kit is designed for the qualitative assessment of neutralising antibodies to wild-type SARS-CoV-2. In-house data shows that different SARS-CoV-2-positive patient sera is able to variably neutralise binding:
![RBD-ACE2 Kit Data [Landing Page] RBD-ACE2 Kit Data [Landing Page]](https://thenativeantigencompany.com/wp-content/uploads/2021/04/RBD-ACE2-Kit-Data-Landing-Page-2.png)
NTRL assay: Binding rate (B/B0%) for negative samples is ≥ 60%. Binding rate for strongly positive samples is ≤ 50%. Borderline samples, with moderate neutralization, may be assessed with binding rates between 50-60. Negative and positive control antibodies were included. SARS-CoV-2 serum samples #1, #2 and #3 are < 50% and considered strongly positive indicating SARS-CoV-2 neutralizing antibody is present in these samples. Samples #4, #5 and #6 are derived from SARS patient sera, and show binding of >50%, indicating moderate or negative neutralizing capacity.
See Our Full SARS-CoV-2 Range
In addition to our neutralisation kits, we offer an extensive range of reagents to support research into the coronaviruses, including antigens, antibodies, receptors enzymes and custom development services. For more information, click the tiles below:
